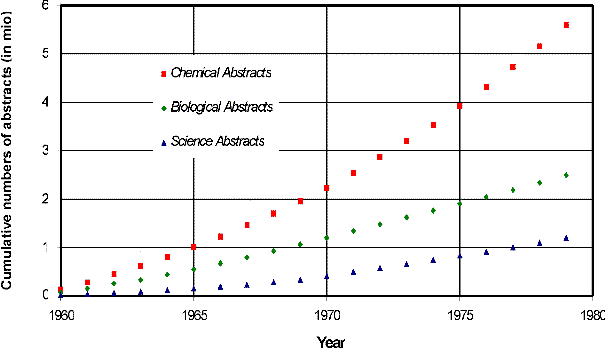
Fig. 1. Cumulative numbers of abstracts (1960-1979):
Chemical Abstracts, Biological Abstracts, Science Abstracts (physics
& electrical engineering!); data from Tague et al. 1981.
HYLE--International Journal for Philosophy of Chemistry, Vol. 3 (1997), pp. 81-94:
Abstract: Part I presents a quantitative-empirical outline of chemistry, esp. preparative chemistry, concerning its dominant role in today's science, its dynamics, and its methods and aims. Emphasis is laid on the poietical character of chemistry for which a methodological model is derived. Part II discusses standard distinction between science and technology, from Aristotle (whose theses are reconsidered in the light of modern sciences) to modern philosophy of technology. Against the background of results of Part I, it is argued that all these distinctions fail, because the underlying concepts of science are either out-dated, one-sided, or arbitrary. A deeper understanding of today's sciences requires, in particular, a philosopical investigation of chemistry.
Keywords: preparative chemistry, science and technology, poietical
science, Aristotle.
Among those who recently turn their philosophical attention to chemistry there is a consensus that chemistry was hitherto a philosophically neglected field. Modern philosophy of science since the days of Whewell, Duhem, Mach and others was mainly philosophy of physics, even when it appeared as General Philosophy of Science. While it remains difficult to prove that such a neclect has always a negative influence on the development of sciences like chemistry, it certainly has a negative influence on our general understanding of science. In order to clarify this I will have a closer look on standard distinctions between science and technology. It is my suggestion that chemistry undermines these distinctions because of serious defects in our general understanding of science.
But first it appears to be necessary to clarify what we mean by chemistry - keeping away philosophical conceptions of science as far as possible. For that reason some empirical data about chemistry are presented in Part I. Though an empirical approach to science cannot, in my opinion, replace philosophical reflection, the latter ought to be in some coherence with the former to prevent us from one-sided and misleading speculations.
Let us first have a quantitative look at the activities of three main natural sciences: physics, biology, and chemistry (earth sciences are not considered here, since their activity is comparably small). By 'activity' I simply mean the production of scientific papers indexed by the respective abstract journals: Science Abstracts (physics & electrical engineering), Biological Abstracts, and Chemical Abstracts.

Fig. 1. Cumulative numbers of abstracts (1960-1979):
Chemical Abstracts, Biological Abstracts, Science Abstracts (physics
& electrical engineering!); data from Tague et al. 1981.
If we compare the cumulative numbers of abstracts from 1960 to 1979 (Fig. 1), there is no doubt that chemistry is by far the most active science. In fact, it is four times higher than physics (& electrical engineering) and nearly two times higher than biology.
Hence, if we talk about our actual sciences, we should (from the quantitative perspective) first and foremost turn our attention to chemistry.
Today there are nearly 3 million chemists all over the world producing some 700 000 papers a year. You may raise the question: what are all these chemists doing? Do they invent and test new theories? Far from it! The answer is: Chemists produce new substances; only last year they made 1.3 million new ones.[2] Figure 2 presents a survey of chemical substance productivity during the past 200 years.
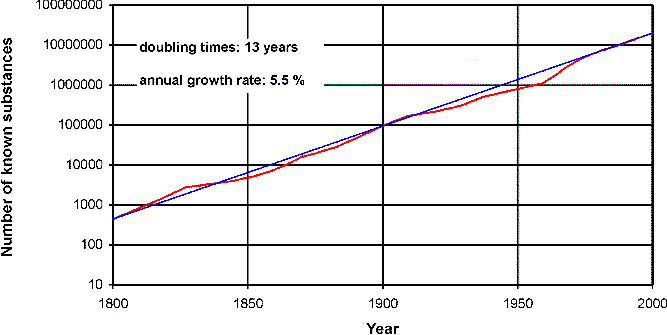
Fig. 2. Growth of chemical substances (data from Schummer 1997a).
Please notice, that the figure has a semi-logarithmic scale. The red (bold) line is the growth of all chemical substances. There is nearly stable exponential growth during the whole period, with annual growth rate of 5.5 % and doubling times of 13 years. (The blue/straight line corresponds to ideal exponential growth and is generated by a fit to the last 20 years). And moreover, there is no saturation until today, as Derek de Solla-Price (1961) suggested for scientific growth in general.[3] Notice that some 95 % of all known substances are artifacts, i.e., you cannot find them in nature.
Producing new substances is, for sure, not the only activity of chemists. Analytical chemists improve analytical methods, quantum chemists try to solve Schroedinger equations, physical chemists measure chemical reactions, technological chemists develop and improve new industrial processes, and so on. But the great majority - about two thirds - actually produce new substances.
You may wonder, why chemists are doing that. A sample survey of 300 papers of one of the most important international journals on general chemistry (Angewandte Chemie) should give a rough answer. Screening out all the reasons (given on demand of the editor) what the production of new substances should be good for provides the results presented in Figure 3:
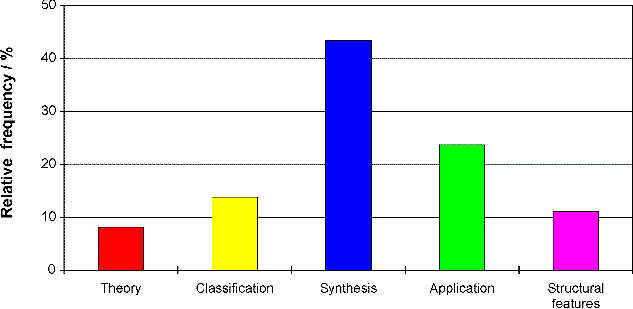
Fig. 3. Relative frequencies of aims in preparative chemistry,
300 journal papers of the years 1980-1995 considered (data from Schummer
1997b).
The aims are divided up into 5 groups. The first group, theory, contains everything philosophers traditionally told us, why experiments are carried out in science: testing, exhausting, refining theories, models, and so on. Theories are, apparently, not very important in preparative chemistry. A bit more important are questions concerning classification, e.g., development of new substance classes, undermining former classificatory distinctions, and so on. (Remember that chemistry had always classificatory problems; nowadays it has to manage more than 16 million substances.) Today's chemists have also a comparable fable for structural features of their substances (strange angles, unusual symmetries, and so on), which is difficult to understand from outside of chemistry. More important, however, are the two remaining groups. The application group contains the search for new materials that might be of practical or technical use, e.g., in pharmacy, agriculture, electro-engineering, and so on. Though applied research is of considerable importance in chemistry, it is not at all the first aim. Instead the great majority of preparative research is performed to improve the preparative abilities of chemistry itself. The synthesis group contains the production of new important reagents or catalysts and the development of new general preparative methods both on empirical and theoretical level.
As the most striking result, nearly half of preparative chemistry can be considered as producing new substances in order to improve abilities to produce more new substances. That is, producing new substances is actually an end in itself here - and an extremely successful one as the exponential growth of substances demonstrates.
Some more detailed results encourage to develop a rough methodological model[4] (Fig. 4) of what might be called 'pure preparative chemistry', which is approximated especially in organic chemistry (some 60%):
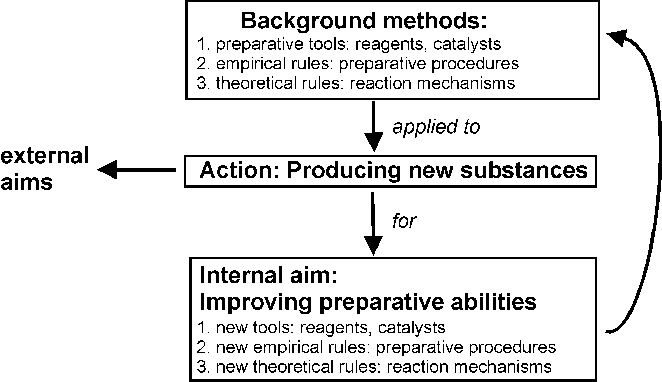
Fig. 4. A methodological model of 'pure' preparative chemistry.
According to this idealized model, the production of new substances occurs
first of all to improve preparative abilities:
The improved abilities actually serve to produce further new substances, in order to improve preparative abilities further, and so on.
Now, we are well prepared to reconsider standard distinctions between
science and technology.
The most famous reflections on science and technology were made by Aristotle.[5] He considered science and technology both as kinds of teachable knowledge looking for reasons on a general level ('know-why'). But he drew four important distinctions that are frequently repeated until today, giving them a kind of common sense status. Therefore it seems to be important to reconsider the original passages in the light of today's science.
According to Aristotle's first distinction, science (episteme) is about the unchangeable, while technology (techne) is about the changeable.[6]
There is no doubt, that chemistry is about changeable things. In fact, substantial change, i.e., the change of chemical substances by chemical reaction is the very essence of chemistry.[7] So according to this criterion, chemistry would be a kind of technology.[8] But notice that the same would go for high energy physics, modern cosmology, biology, geology, and so on. In short: nearly all modern sciences are about changeable things and should be considered as technologies. Hence, this criterion seems to be not very useful.
On the other hand, Aristotle's own science (episteme) of nature is also about changeable things,[9] in contrast to mathematics and his 'theology', which are about the unchangeable. The puzzle may be solved only by distinguishing between two types of objects: while the empirical objects of nature are changeable, their principles of motion remain unchangeable. If we want to make sense of Aristotle's distinction between science and technology, we have to turn our attention to the principles of motion of natural objects and artefacts.
Aristotle distinguishes between theoretical science (theoretike) and poietical science (poietike) with regard to the objects' different principles of motion.[10] The objects of Aristotle's physics (as a theoretical science) bear their own principle of motion,[11] i.e., they are moved or generated by their own inherent forces that remain always the same. The objects of techne or poietical science, on the other hand, have their principle of motion from without, i.e., they are moved or generated by the technician's activity, according to his aims that may change from day to day.
This distinction clearly assigns chemistry the status of a poietical science, since most of its objects are generated by chemists as we have seen in Part I. Chemists may perfectly understand the causes of chemical change. However, they lose their status as scientists of nature by the slightest intended intervention, by disturbing the inherent principle of motion of their objects.[12]
Two objections rise. First, the metaphysical distinction based on the concept of principle of motion has been dropped since the dawn of mechanistic philosophy. Physicists do not care, whether their objects are of artificial or natural origin as long as these are describable in terms of physical laws. While motions may be explained in terms of causes, Aristotle's 'principle of motion' lost any meaning in science. Secondly, and more important, generating phenomena in artificial contexts is the very essence of the experimental method. There is, for instance, no electric currency to measure, as long as we do not apply an electric field. While Aristotle's concept of theoretical science is bound to what Dewey (1917: 41) once called the 'spectator notion of knowledge', this has been discarded in most sciences for centuries - in chemistry/alchemy, to be sure, for millenia. Hence, since according to Aristotle's criterion all experimental science would be technology, we should better drop it today.
Aristotle drew another distinction: Starting from sensations of concrete things, science finally aims at general knowlege, whereas technology goes one step further and applies general knowlege back to concrete things.[13]
The latter seems to be again a distinguished characteristic of preparative chemistry, as we have seen in Part I. Chemists apply general chemical knowlege to change concrete material samples. But we should be careful here again, because our modern concept of science is less optimistic and methodologically different to Aristotle's concept. Since Bacon we do not believe any longer in Aristotelian inductivism. General knowledge always remains preliminary knowlege, subject to empirical falsification. As soon as a general law is born, we try to apply it to new experimental arrangements, i.e., to concrete things, in order to test or to exhaust the law. Moreover, once a general law has achieved a more trustworthy status, we tend to use it for new instrumental skills. It is actually hard to imagine modern science simply stopping at a certain general law, and enjoying its putative truth without applying it to concrete things.
Aristotle's own preference of such a kind of science (the highest form of happiness (eudaimonia) in using one's dianoetical virtues) leads to the next point.
Aristotle's last approach draws a distinction between different kinds of activity. Scientists look for theoretical knowlege (theoria), that is an activity having an end in itself (and as such being a candidate for the highest form of happiness).[14] Technicians, on the other hand, produce new things (poiesis), and such an activity has always an end in something else.[15] In other words: the purpose of scientific activity is just that activity itself, whereas poietical activity is always good for something else.
Producing new chemical substances is, to be sure, a poietical activity par excellance. Moreover, the production of new substances is usually intended to be good for something else (see Part I). Consequently, preparative chemistry would be a technology par excellance. However, as we have seen above, the aim of producing new substances is, for the most part, to improve the abilities of the field. Hence, though the individual poietical activity has not an end in itself, it has an end in its own field: supporting the scientific community. Modern ('big') science is a complex network of cooperative research based on the division of labour that was quite unknown to Aristotle. If we do not consider that, every modern science would again be technology according to Aristotle.
It should be emphasized, that ordinary distinctions between different 'qualities' of aims, say, between 'practical use' and 'understanding the world',[16] do not lead back to Aristotle's point. As soon as a certain activity is instrumentalized, which is necessarily the case in cooperative research of 'big science', it definitely loses its character of having an end in itself (and misses Aristotle's ideal of happiness of bios theoreticos).
In the light of modern 'big science', we might be willing to reformulate Aristotle's distinction: if the activity aims to contibute to its own field, it is science; if the end is outside of the field, it is technology. But even that runs into trouble, since, first, we have interdisciplinary research undermining the field distinction. And, secondly, scientific fields are usually heterogeneous and intricate with regard to ends; some, for sure, always get outside of the field.
So we may conclude that Aristotle's four distinctions between science and technology, though frequently repeated in various combinations until today, fail, because the structure of science has basically changed since the ancients. Today's science (1) is about changeable things, (2) is mostly experimental science, (3) follows a different methodology, and (4) is 'big science' in the sense of complex research cooperation based on the division of labour.
More recent philosophers have claimed some further methodological differences between science and technology.[17]
Scientists, it is said, use isolating and abstracting methods to discover very general laws. Engineers, on the other hand, apply multi-factorial models to approach concrete and complex systems. However, that sounds rather like a dinstinction between physics and chemistry.[18] In fact, chemists are concerned with developing multi-factorial models to approach the concrete and complex systems of chemical substances. Every substance is different, and it is just the difference that chemists are interested in. But you can even find the same kind of approach in biology, mineralogy, geology, meteorology, and the rest. What is wrong with that distinction is that it mixes up traits of theoretical physics with science in general.
It is sometimes stated that scientist try to understand a process in principle only, while engineers try to optimize the process for certain aims. However, even that distinction runs into trouble with regard to preparative chemistry. In fact, a great deal of work is done in chemistry to improve preparative procedures. Chemists are very busy to optimize the yield of their preparation according to theoretical standards. The higher the yield, the better the scientific result. The reason behind that is quite simple: Modern science has a cooperative structure. The outcome of a certain research serves as a means for later research and is, consequently, subject to optimizing.
Optimizing strategies are not restricted to chemistry. You can find the same even in physics, especially in solid state physics. Remember, for instance, the field of superconductivity, where the physics Nobel Prize was awarded not for a new theory, but for materials with the hitherto highest temperature superconductivity. Moreover, there is an inherent trend of physics to extend experimental conditions to extremes: the highest/lowest temperature, the highest/lowest pressure, and so on.
So again this distinction fails because of a one-sided misconception of science.
There is an old and famous struggle among philosophers, whether scientific innovations should be termed 'discovery' or 'invention'. According to the discovery paradigm, chemists discover new changeabilities, e.g., that substance A changes to B under conditions C. According to the invention paradigm, chemists invent new procedures, e.g., producing substance B from A under conditions C. These different paradigms are frequently used to distinguish between science and technology: science discovers, technology invents.[19] From that it is derived that there would be different logical types of results: science produces law-like statements, technology produces rule-like statements.[20]
I am afraid that this criterion does not work at all to distinguish between actual activities. For the decision is not based on empirical evidence about certain scientific activities. It is rather meta-methodologically rooted in a priori assumptions of the philosophical approaches themselves: empiricists (in the analytic tradition) prefer the discovery paradigm, constructivist (of any color) prefer the invention paradigm. Due to the empirical arbitrariness of these assumptions, so called empiricists are able to reconstruct any activity as science, while constructivists, on the other hand, may claim that the same activity is technology. The flaw of reconstructivism - and the reason for endless debates in philosophy - is that there is no accepted meta-criterion for correct reconstruction.
However, if we are actually interested in empirical evidence, we should turn our attention to the linguistic form of scientific reports. There are indeed some perceptive linguistic studies on chemistry papers and textbooks[21] giving the impression that the use of rule-like or law-like statements is just a temporarily fashion, a facon de parler. In experimental sciences like chemistry rule-like and law-like statements seem to be pragmatically (though not syntactical) equivalent, in the sense that every rule-like statement is easily translated into a single law-like statement, and vice versa, without losing information or bothering about different formal calculi.[22]
I first presented some facts about chemistry as today's most active scientific field, in order to point out the poietical character even of 'pure' chemical research. In the light of this, all standard distinctions between science and technology seem to fail, because the underlying concepts of science are either out-dated, one-sided or arbitrary (Table 1). Today's sciences are much more complex and heterogeneous than philosophers are still willing to admit, and the same probably goes for the relationship to technology too. Philosophers of science have nearly neglected what Kuhn (1977) once called the 'Baconian Sciences' covering more than 90% of today's science, i.e. (beside the various sub-disciplines of chemistry) solid state physics and the whole of material sciences, molecular biology, genetics and genetical engineering, pharmacy and biomedical sciences, mineralogy, petrology and the major part of earth sciences, and so on. I claim that all these fields are more closely related to chemistry than to (the philosophical image of) physics in nearly every aspect. That is why a philosophy of chemistry is in need to achieve a deeper, more realistical understanding of our actual sciences.
|
Difference in |
Science |
Technology |
Criticism |
| Object I |
unchangeable |
changeable |
out-dated concepts of science, ignoring: - dynamic ontologies |
| Object II | principle of motion inside | principle of motion outside | - experimental method |
| End | knowing the general | applying knowlege to the concrete | - modern methodology |
| Activity | theoria: end in itself | poiesis: end in something else | - modern "big" science (division of labour) |
|
Method I |
abstraction, idealization |
approaching complex concrete systems |
one-sided concepts of science, ignoring: - varieties of scientific methods |
| Method II | recognizing processes in principle | optimizing processes in detail | - varieties of scientific methods |
| Innovation |
discovery |
invention |
arbitrary concepts of science, ignoring: - meta-methodological presuppositions |
| Logical type of result | law-like statements | rule-like statements | - pragmatical irrelevance, empirical non-significance |
* A first draft of this paper was read at the Academic Session 1997 of the Académie Internationale de Philosophie des Sciences, Karlsruhe, Germany, May 19-24.
Aristoteles: 1984, The Complete Works of Aristotle, ed. by J. Barnes, Princeton University Press, Princeton.
Bacon, F.: 1858, The Works of Francis Bacon, coll. and ed. by J. Spedding, R.L. Ellis, D.D. Heath, vol. I, London 1858.
Bartels, K: 1965, 'Der Begriff der Techne bei Aristoteles', in: H. Flashar, K. Gaiser (eds.), Synusia. Festgabe für W. Schadewaldt, Neske, Pfullingen, pp. 275-87.
Beier, R.: 1977, Untersuchungen an amerikanischen und britischen Fachtexten der Chemie: Analyse finiter Verbformen unter besonderer Berücksichtigung der Konstruktionen des Typs 'be + Perfektpartizip', Lang, Frankfurt/M. et al.
Bunge, M.: 1966, 'Technology as Applied Science', Technology and Culture, 7, 329-347 (reprinted in: Rapp 1974, pp. 18-39).
Bunge, M.: 1972, 'Toward a Philosophy of Technology', in: Mitcham/Mackey 1972, pp. 62-76.
Bunge, M.: 1988, 'The Nature of Applied Science and Technology', in: Philosophy and Culture. Proceedings of the XVIIth World Congress of Philosophy, ed. by V. Cauchy, vol. II, pp. 599-604.
Chemical Abstract Service: 1997, CAS Statistical Summary 1907-1996, Columbus/Ohio.
Chen, Shing-lung.: 1995, Pragmatik des Passivs in chemischen Fachkommunikationen: empirische Analyse von Labordiskursen, Versuchsanleitungen, Vorlesungen und Lehrwerken, Lang, Frankfurt/M. et al.
Dewey, J.: 1917, John Dewey. The Middle Works, vol. 10, Southern Illinois Univ. Press, Carbondale.
Düring, D.: 1944, Aristotle's Chemical Treatise: Meteorologica Book IV, Göteborg (reprint: New York-London 1980).
Hacking, I.: 1983, Representing and Intervening, Cambridge Univ. Press, Cambridge.
Kline, R.: 1995, 'Construing "Technology" as "Applied Science"', Isis, 86, 194-221.
Kuhn, T.S.: 1977, The Essential Tension, Univ. of Chicago Press, Chicago.
Lelas, S.: 1993, 'Science as Technology', The British Journal for the Philosophy of Science, 44, 423-442.
Lenk, H.: 1973, 'Zu neueren Ansätzen der Technikphilosophie', in: Lenk/Moser 1973, pp. 198-231.
Lenk, H.; Moser, S. (eds.): 1973, Techne - Technik - Technologie. Philosophische Perspektiven, Verlag Dokumentation, Pullach.
Lenk, H.: 1980, 'Technik und Wissenschaft' and 'Wissenschaftstheoretische Probleme der Technikwissenschaften', in: J. Speck (ed.), Handbuch wissenschaftstheoretischer Begriffe, Vandenhoeck, Göttingen, vol. 3, pp. 623-32.
Mitcham, C.; Mackey, R. (eds.): 1972, Philosophy and Technology, Free Press, New York.
Price, D.J. de Solla: 1961, Science Since Babylon, New Haven and London, Yale University Press.
Rapp, F.: 1973, 'Technik und Naturwissenschaften - eine methodologische Untersuchung', in: Lenk/Moser 1973, pp. 108-32; English reprint in Rapp 1974, pp. 93-114.
Rapp, F. (ed.): 1974, Contributions to a Philosophy of Technology, Reidel, Dordrecht.
Rapp, F.: 1996, 'Technik und Naturwissenschaft', Ethik und Sozialwissenschaften, 7, 423-34.
Ropohl, G.: 1996, 'Das Ende der Natur', in: L. Schäfer, E. Ströker (eds.), Naturauffassungen in Philosophie, Wissenschaft, Technik, Bd IV: Gegenwart, Alber, Freiburg-München, pp. 143-63.
Schummer, J.: 1994, 'Die Rolle des Experiments in der Chemie', in: P. Janich (ed.), Philosophische Perspektiven der Chemie, B.I., Mannheim, pp. 27-51.
Schummer, J.: 1996a, Realismus und Chemie. Philosophische Untersuchungen der Wissenschaft von den Stoffen, Königshausen & Neumann, Würzburg.
Schummer, J.: 1996b, 'Die stoffliche Weltveränderung der Chemie: Philosophische Herausforderungen', in: Proceedings of the XVIIth German Congress of Philosophy, ed. by C. Hubig, H. Poser, Leipzig, vol. I, pp. 429-436.
Schummer, J.: 1997a, 'Scientometric Studies on Chemistry I: The Exponential Growth of Chemical Substances, 1800-1995', Scientometrics, 39, 107-123.
Schummer, J.: 1997b, 'Scientometric Studies on Chemistry II: Aims and Methods of Producing new Chemical Substances', Scientometrics, 39, 125-140.
Schummer, J.: 1997c, 'Towards a Philosophy of Chemistry', Journal for General Philosophy of Science, 28 (forthcoming).
Schummer, J.: 1997d, 'Physical Chemistry - Neither Fish nor Fowl?', in: P. Janich, N. Psarros (eds.), The Autonomy of Chemistry, Königshausen & Neumann, Würzburg (forthcoming).
Tague, J.; Beheshti, J.; Rees-Potter, L.: 1981, 'The Law of Exponential Growth: Evidence, Implications, and Forecasts', Library Trends, 30, 125-150.
Tiles, J.E.: 1993, 'Experiment as Intervention', The British Journal for the Philosophy of Science, 44, 463-75.
van Brakel, J.: 1997, 'Chemistry as the Science of the Tranformation of Substances', Synthese, 111, 253-282.
Copyright Ó1997 by HYLE and Joachim Schummer