http://www.hyle.org
Copyright © 2017 by HYLE and Alastair Iles, Abigail Martin, and Christine Meisner Rosen
Undoing Chemical Industry Lock-ins: Polyvinyl Chloride and Green ChemistryAlastair Iles, Abigail Martin, and Christine Meisner Rosen*
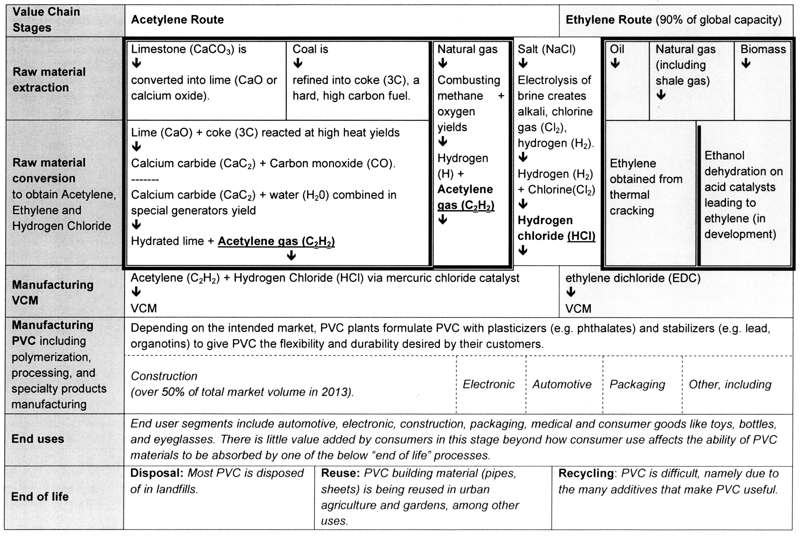
1. IntroductionPolyvinyl chloride (PVC) is one of the oldest and most ubiquitous plastics in the world, dating back to the 1920s. In terms of global production volumes, PVC is second only to polyethylene. In 2015, manufacturers produced 43.6 million tons, worth $US 57 billion (Zion Research 2016). Over 50 percent goes to make infrastructure materials like water pipes, wire coverings and window frames used in buildings and automobiles, with the rest used to create durable consumer products such as toys, credit cards, and vinyl curtains. PVC has replaced many traditional materials like textiles and wood because it offers longevity and strength. While PVC presents an ‘old’ chemistry issue, in that its environmental harms have been fought over for well over 40 years, it still presents widespread risks to health and ecosystems through its myriad contemporary uses. In the past 20 years, green chemistry has emerged as a powerful new philosophy for designing molecules, reactions, and products to be intrinsically non-toxic and sustainable. Many green chemists such as Terry Collins and John Warner envisage a world where chemists and engineers – along with company managers and government regulators – take the ethical lead in diminishing the exposure of human societies to harmful chemicals. These scientists argue that green chemistry is founded on the precautionary principle. Put briefly, this ethical principle holds that companies, engineers, and chemists should act to prevent chemical risks even if these are scientifically uncertain, and even if regulation does not require action. As a result, companies can benefit in many ways: they no longer need end-of-pipe technologies to control pollution from chemical processes, and can avoid exposing people to toxic risks through consumer products. Nonetheless, companies and scientists can reject the precautionary principle, or construe it in divergent ways. The chemicals used to make PVC – from chlorine to mercury and from phthalates to vinyl chloride monomers – are known to cause cancers, neurological disorders, reproductive and development problems, and other deleterious health effects. Some effects are well-established while others are debatable. Some chemists say that if PVC had been developed more recently than the 1930s, it would never have been commercialized. Still, we live in a world with PVC, whose impacts must be dealt with somehow. Green chemists are creating solutions to tackle PVC impacts (or even to replace PVC altogether) – yet they have run into a fundamental barrier: the pervasive technological and economic lock-ins of PVC. As PVC has matured, various production processes and consumption patterns have become intertwined with each other, to the point where they are hard to unwind. The PVC case thus raises three major ethical issues. Should the chemical industry overcome the inertia of path dependent technologies and introduce safer, more sustainable technologies? What will motivate companies and their employees to practice green chemistry under conditions where changing technologies and businesses can create substantial economic, market, and technical risks? How should the precautionary principle be applied in terms of the real-world complexities of manufacturing chemicals? To address these issues, we examine how green chemistry can help generate new path-shaping opportunities throughout the PVC lifecycle. We briefly review the historical evolution of the PVC production chain to show where and how the many lock-ins that characterize this chemical have materialized over time. We then review two key stages in the PVC lifecycle – feedstock production and PVC manufacturing for end-uses – to illustrate some of PVC’s environmental and health impacts, along with green chemistry solutions that could be adopted. We conclude with analysis of the considerations that key groups of companies may face when choosing whether to do green chemistry research and development. As you read through the PVC background, you can reflect on the ethical considerations that should come into play when deciding whether and how to change a deeply entrenched chemical chain. 2. The Precautionary Trajectory of Green ChemistryThe precautionary principle can be traced back to the Vorsorgeprinzip idea that developed in Germany during the early 1970s (European Environmental Agency 2001). When protecting water from pollution, German officials thought, there ought to be ‘forward-looking’ planning to prevent environmental damage. In retrospect, much of the US environmental law framework that emerged around 1970 was founded on the precautionary principle (Raffenberger & Tickner 1999). For example, the Clean Air Act requires the Environmental Protection Agency to protect public health and welfare when making ambient air quality standards for pollutants such as lead and nitrogen dioxide. In contrast to subsequent laws, regulators cannot consider economic cost in setting standards. European countries were slower to put the principle into practice but it is now firmly established in EU environmental laws. By the 1990s, the precautionary principle was being widely cited in international environmental treaties and declarations. Within the US, the Reagan Administration greatly weakened the emphasis on prevention during the 1980s, by requiring a favorable cost-benefit analysis (a quantitative method to determine whether benefits exceed costs) before approving new rules (Ashford 2005). Its officials prioritized voluntary industry action over regulation. By the 1990s, government agencies and courts were repeatedly accepting the arguments of industry lobby groups (e.g., the Chemical Manufacturers Association) that policy interventions without a strong economic case needlessly damaged businesses. In this context, a group of 32 lawyers, activists, and scientists met in January 1998 at the Wingspread Conference Center in Racine, Wisconsin to discuss how to define the precautionary principle for environmental health decision-making. These participants issued a consensus declaration known as the Wingspread Statement on the Precautionary Principle (Tickner et al. 2003). The statement said: When an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause-and-effect relationships are not fully established scientifically. The conference affirmed that whoever wants to create the potential harm must have the burden of proving that it is not injurious (because the actor often has much more capacity and knowledge to do so compared to, say, the public; and may benefit lucratively from the activity). If more scientific evidence is needed to determine whether a proposed activity is safe for the public, the actor must generate this knowledge. A reasonable range of alternatives to the activity (including no action) should be evaluated, while decision-making should be "open, informed and democratic, and must include potentially affected parties" (ibid.). Advocates commonly invoke reasons such as the following to justify the precautionary principle (European Environmental Agency 2001). Given data limitations, scientists may not be able to conclusively prove that something causes an effect. Achieving corroboration may require decades of scientific research and in the meantime, substantial, irreparable harm could be caused to societies and ecosystems. Yet, taking early action may save human lives, not to mention large sums of public funds through avoiding health care and environmental clean-up costs. Existing regulatory practices like risk assessment have failed to adequately protect humans and animals.[1] Traditionally, risk assessment has assumed that chemicals can have ‘safe’ levels of exposure, only to be confounded by emerging scientific knowledge. Moreover, uncertainties may be due to the existence of ‘undone science’, or gaps in technical knowledge because of a long-running failure of industry and scientists to inquire into, for example, the health effects of pesticides on farm workers (Frickel et al. 2009). In industrial countries like the US, dominant policy frameworks tend to be utilitarian. Consequentialist ethics emphasizes looking at the consequences of an action to decide if it is morally permissible (Martin et al. 2016). When invoking utilitarianism, people say that the morally right action is the one that produces the most utility (‘the greatest good for the greatest number’). Cost-benefit analysis (CBA) is premised on the idea that government rules or industry decisions should proceed only if significant benefits (calculated in monetary terms) will result. For example, a company should only choose to remove a chemical if a large number of people will have their health protected without excessive cost or loss of profit. Policies that force industry to spend many millions of dollars per human life preserved or enhanced are economically ‘inefficient’. Critics have pointed out that CBA conceals numerous problems, from imposing artificially monetary prices on environmental health to relying on fallacious, often ideologically driven assumptions about the value of human life and health (Ackerman 2008). Nonetheless, this way of thinking continues to be tremendously influential in the green chemistry arena, since it meshes with traditional business worldviews. Companies can, and do, interpret the precautionary principle in a more utilitarian sense. They say that there are limits on what should be done – that precaution only ‘works’ beyond a certain threshold of danger that would warrant costly interventions. For them, many sorts of uncertainty do not pass this line – because of their underlying organizational decision-making criteria. By contrast, strong interpretations of the precautionary principle invoke deontological ethics, particularly in the European Union. That is, moral action depends on the intention: whether an action is done for the right reasons. Deontologists assert that people have a duty to do the right thing no matter the consequences. The Wingspread Statement bases precaution on a paramount duty to prevent harm to humans and ecosystems, and does not qualify precautionary measures as worthwhile only if they are cost-effective. Crucially, who decides on what constitutes an actionable peril is not industry but the societies and peoples who are being potentially harmed. This shift of judgment power recognizes that corporations tend to prioritize their own existential interests over those of societies. Companies should, then, eliminate risks that societies see as particularly damaging (e.g., harming the cognitive development of children) even if conventionally calculated monetary benefits may not be large,[2] and even if substantial uncertainties exist. Do chemists, engineers, managers, designers, and many others involved in chemical production and use have a special responsibility to repair the toxicity and sustainability of molecules? Must they be constrained by perceptions of their own capacity for action and by the findings of cost-benefit analyses when deciding how far they can go? Must they integrate societal views into their decision-making? In short, how can the precautionary principle be applied to chemistry? In 1998, chemists Paul Anastas and John Warner proposed one way to operationalize the precautionary principle: green chemistry. They defined green chemistry as "The utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products" (Anastas & Warner 1998, p. 2). While green chemistry originally began as a specific pollution prevention practice (i.e., molecular design to reduce waste), it quickly evolved conceptually into a much broader set of approaches targeting toxicity and sustainability (Geiser 2015). Green chemistry seeks to inject ecological and health values into the otherwise technical process of designing and making chemicals. While many green chemists underline the fact that no chemical can ever be fully benign, they prioritize these values much more than had been the case before. Anastas and Warner (1998) drafted what became known as the 12 Principles of Green Chemistry (12 GC, see Table 1). Arguably, this framework is grounded in the deontological version of the precautionary principle. The traditional model of risk suggests that Risk = Hazard x Exposure. Instead of simply containing exposures to risk, Anastas and Warner argued, chemists should focus on preventing hazards to begin with. They suggested that prevention ‘at the source’ is superior to simply controlling pollution and waste, since if there is no hazard, then there is no risk. The 12 GC principles are meant to inspire molecular designers in developing methods and technologies to create inherently benign materials and energy. These principles can also guide the decisions of a broad community of process and product designers, business managers, regulators, and advocacy groups. The 12 GC principles call on chemists to engage in various practices, such as choosing less hazardous reagents and solvents, or designing reactions to have ‘atom economy’ (or efficient conversion). Chemists should design molecules to degrade readily in the environment, draw on renewable feedstock such as agricultural crops, and integrate catalysts to improve reaction productivity. Importantly, the principles have been updated to say chemists should design safer chemicals. This implies replacing harmful chemicals with better ones and conducting alternatives analysis to know which ones these are.
Table 1: The Principles of Green Chemistry (from Anastas & Warner 1998). Early on, leading green chemists acknowledged that they have special moral agency to reshape the molecules that they help make. Anastas and Williamson (1996, p. 1) wrote: For those of us who have been given the capacity to understand chemistry and practice it as our livelihood, it is and should be expected that we will use this capacity wisely. With knowledge comes the burden of responsibility. Chemists do not have the luxury of ignorance and cannot turn a blind eye to the effects of the science in which we are engaged. Academic chemist Terry Collins considers it an ethical imperative for his peers to create what he calls a ‘sustainable civilization’ (Collins 2001). Many green chemists point to the terrible, known harms of lead, polychlorinated chemicals, asbestos, and other substances as validating their work (Tickner & Geiser 2005). They believe there is an overwhelming case for the removal of such substances. By contrast, green chemists (who are still only a small minority in the chemistry profession) can differ as to whether more scientifically uncertain effects should be enough to justify using specific green chemistry choices. Even so, by 2001, some chemists were already saying: "Our present knowledge strongly suggests that anthropogenic [endocrine disrupters] should be identified and eliminated altogether" (Collins 2001, p. 49). They feel responsible for having collectively helped create a planet where households are filled with chemicals, where wildlife bear heavy burdens of persistent organic pollutants, where girls are maturing much younger due to endocrine disruption. More generally, scientists often must work with some degree of uncertainty as they make particular green chemistry decisions. For example, using computer modeling tools to predict the toxicity of a new substance means accepting a level of uncertainty in these tools because of inadequate health data and modeling assumptions (Faulkner et al. 2017). Such ethical questioning is also entering the downstream consumer product industry. Product developers at the Seventh Generation firm argue: "From the perspective of a green household cleaning formulation the risk of harming human health and/or the environment far outweighs the benefit of providing consumers with the latest innovation in household cleaning" (Bondi 2011, p. 430). They suggest, however, that green chemistry does not mean that companies cannot innovate: green chemistry is intrinsically innovative since it creates safer cleaning products that exclude endocrine disrupters. Products must be judged according to a new, socially made criterion of safety, alongside performance and cost. This criterion should include precaution where necessary. If firms are wavering about what to do, the precautionary principle offers a way to rank priorities for change (Bondi 2011), depending on the gravity and magnitude of the potential danger, populations who may be affected, and the reversibility of the danger. The emphasis is on hazard, not on cost. To accomplish this ranking, interest in the analysis of alternatives is growing: firms can compare between different solutions, including green chemistry, to choose the safest alternative (Tickner & Geiser 2004). Importantly, American and European actors can diverge in their understandings of whether and how the precautionary principle underlies green chemistry, because of their political, cultural, and historical conditions (Wilson & Schwarzman 2009). In Europe, precaution is partially built into the REACH regulatory framework that has governed chemicals in this region since 2006. Companies must prove that their products, whether new or existing, are safe; substances that are likely to pose significant perils can be de-registered after regulatory review. To some degree, then, firms have strengthened their green chemistry efforts as a regulatory compliance approach. In the US, green chemistry has largely occurred through voluntary industry actions, because a dysfunctional toxics regulatory state prevailed until July 2016, permitting lax oversight of chemicals already on the market. Here, the precautionary principle has likely played a greater role in motivating ethical industry action in the absence of stringent regulation. In this paper, we look more at the latter situation because here ethics becomes central. 3. Historical Development of PVC Industry Lock-insTo understand how PVC became widely used and locked-in, we look at the history of industry choices in manufacturing PVC. More generally, the ability of companies to use green chemistry principles may depend on the ways in which technologies and reactions have evolved. 3.1 Early PVC productionThe first generation of PVC polymers emerged in Germany but was unsuccessful in the market due to performance problems. In 1872, a German chemist named Baumann first discovered how to polymerize vinyl chloride monomers (VCM) into PVC. Commercial production, however, did not accelerate until 1913, when another German chemist called Klatte patented a new method for producing VCM. This method obtained acetylene feedstock by reacting calcium carbide with water in special generators, and adding hydrogen chloride gas to acetylene gas using a mercury chloride catalyst (Wilkes et al. 2005, see Table 2). Klatte’s approach to VCM production was particularly economical because it made use of acetylene and chlorine – raw materials that had encountered problems of overproduction. Beginning in the late 19th century, companies had built chlor-alkali factories to meet rapidly growing demand for alkali (caustic soda, soda ash, sodium hydroxide, and baking soda) (Thornton 2000). Through brine electrolysis, chlor-alkali facilities produced alkali with chlorine and hydrogen as by-products. While chlorine was used as a bleaching material in the textile and paper industries, alkali was the more valuable industrial product due to interest from manufacturers of glass, soap, paper, textiles, and other products. However, for every ton of caustic soda made, 1800 pounds of chlorine were also generated (Thornton 2000). If producers could not find uses for chlorine, they would be forced to slow caustic soda production or store dangerous chlorine gas. Thus, the industry sought markets for chlorine-based products, including VCM made from acetylene and chlorine as raw materials. PVC production in Germany grew during World War I (Mulder & Knot 2001). PVC was an attractive construction material because it offered a longer life for products traditionally made from corrosion-prone metals. Yet performance problems were soon apparent: PVC degraded when exposed to heat and light, turning brittle. With competition from low-cost, durable natural materials like rubber, interest in PVC collapsed after WWI. 3.2 A second generation of PVC productionIn the 1920s, a second generation of higher quality PVC emerged through innovations in polymer science and process engineering. Many companies founded polymer divisions, igniting the nascent field of polymer science (Chandler 2009). One innovation in particular transformed the performance value of PVC. In 1926, Waldo Semon, a polymer scientist at the US tire manufacturer B.F. Goodrich, discovered that formulating PVC with additional substances turned it into a flexible, water-proof, and fire-resistant material that could bind to metal and be readily molded into stand-alone products. By 1930, B.F. Goodrich was producing PVC commercially, with other companies following in the US, Germany, and Japan. In Germany, I.G. Farben researchers developed a co-polymerization method that promised to soften PVC. Polymer scientists continued to experiment with PVC formulations, adding plasticizers (to make it softer, less brittle) and stabilizers and hardeners (to make it more durable, more rigid). In addition, new developments in PVC process engineering improved the injection molding capabilities of PVC manufacturers. As a result, PVC production expanded once again during and after World War II. For materials that were hard to attain in a wartime economy like electrical wire coatings, PVC became a desirable substitute. After the war, to solve the problem of excess PVC production, PVC manufacturers aggressively marketed their product as a cost-effective, higher performing alternative to other plastics and materials like woods, metals, glass, rubber, and ceramics (Mulder & Knot 2001). Initially, consumers perceived PVC as a sub-standard plastic, since it deteriorated with use. Using additives therefore became the dominant method for controlling PVC characteristics. Additives emerged as a sub-sector in its own right, as industry invested in developing hundreds of additives. Rigid PVC formulations suited mass-produced construction and consumer products, including windows, doors, pipes, combs, toothbrushes, and eyeglass frames. Flexible PVC formulations included flame-resistant cable insulation, water-proof raincoats, shower curtains, artificial leather, and phonograph records. By the 1970s, the average consumption of PVC per person in industrialized countries exceeded 20 pounds yearly. 3.3 The shift from acetylene to ethyleneIn the 1950s, many companies chose between acetylene and ethylene as feedstock for PVC production (Mulder & Knot 2001). The primary method for making VCM was still the hydrochlorination of acetylene gas. In the early 1950s, a new route for producing VCM from ethylene appeared, at a time when ethylene was abundant due to cheap oil supplies. Although ethylene is the raw material for most basic petrochemical products, the industry searched for additional commercial processes to consume ethylene, and turned plastics including PVC into a new huge end-use market (Spitz 1988). The ethylene-EDC-VCM route is depicted in Table 2. Most ethylene comes from petroleum production, although it can also be obtained from natural gas and biomass. This VCM route involves two major processes. The first is the direct chlorination process which reacts chlorine (obtained from salt electrolysis) and ethylene to form the intermediary ethylene dichloride (EDC). The second is the oxychlorination process: hydrogen chloride (HCl) is obtained as a by-product from the direct chlorination process and used to create more EDC by reacting HCl with ethylene in the presence of catalyst and air (or oxygen). The EDC from this process is then dehydrated and thermally cracked using pyrolysis (a thermochemical process of decomposing organic material in high temperatures in the absence of oxygen or halogens) to yield VCM. EDC and VCM can be sold as commodities to PVC producers, but large chemical companies like Dow can house the entire EDC-VCM-PVC chain. Today the ethylene route accounts for more than 75 percent of global PVC production capacity (Wilkes et al. 2005). It is used mostly in the US, Europe, and other countries with well-developed petrochemical industries and strict environmental regulations. Yet, the acetylene route remains attractive in places where oil supplies are costly or scarce and a readily available supply of lime and coke exists to convert calcium carbide into acetylene. Calcium carbide production requires substantial energy resources as well. Most VCM plants in China, Russia, and other parts of Eastern Europe therefore use this process. China’s VCM production is now at least 85% from acetylene (ICIS Chemical Business 2012). Asian PVC factories are particularly polluting and energy-intensive, compared to PVC factories in other regions. Today, the two production pathways for VCM continue to straddle the global value chain for PVC, as shown in Table 2. Each route requires combining a hydrocarbon feedstock (such as ethylene or acetylene) with hydrogen chloride (made from hydrogen and chlorine gas). Over time, both routes have been refined for increased scales of operation and efficiency. Yet, fundamentally new and commercially viable pathways for making PVC have not been developed since the 1950s.Ironically, PVC may have become prevalent because it was one of the first successful commodity plastics back in the 1930s. A polymer scientist wrote in 1966: "Had this polymer been discovered at the present stage of development of the plastics industry, it would almost certainly have been eliminated as useless because of its general instability to all common degradative agents" (Grassie 1996, p. 647). But PVC now drives chlorine production, rather than serving as an outlet. PVC is now the largest downstream product of chlorine gas, accounting for 41% of chlorine demand in the US and 38% in Europe (Thornton 2000). PVC is widely used because of its cheapness and technical advantages. The chemical industry has built a vast production system around the two pathways and many manufacturing industries depend on PVC as a material for their own products (Mulder & Knot 2001, Knot et al. 2001). Many downstream production processes rely on specialized formulations of PVC with modern additives. Numerous users of PVC – for example, builders, water engineers, and home renovators – prefer it for its ease of use and durability (Mulder & Knot 2001). To change or abandon PVC use, companies would have to invest in new technologies, molding machines, and feedstock. They would have to reformulate their products to remove PVC or to use different PVC formulations. Companies are also unwilling to relinquish a highly lucrative market, while their customers are reluctant to switch away from a substance. In these ways, PVC shows how the chemical industry features many lock-ins that reinforce each other and make it difficult to escape from a technological path. However applying green chemistry ideas can open new scope for making PVC production more malleable again. Table 2. The Global Value Chain for PVC.
4. Opportunities for Green Chemistry in the PVC ChainDespite PVC’s many useful applications, labor and environmental advocates, academic researchers, and others have argued that the PVC value chain causes many hazards (Thornton 2000). PVC is one of the most heavily scrutinized chemicals of all. Numerous critics have assailed this substance since the 1970s and industry claims to have studied it thoroughly for its toxicity. Nonetheless, as we will see, extensive evidence of toxicity associated with PVC does exist, though significant uncertainties exist around the additives used in PVC. What, then, is the scope for using green chemistry? What moral agency do various actors within the PVC chain have to use the precautionary principle and therefore justify using green chemistry? We employ global production chain analysis to help identify some opportunities for putting green chemistry principles into practice. The PVC chain begins with raw material extraction. In between, there are a number of manufacturing stages. The end-of-life stages may extend the chain further, depending on how PVC products are disposed of, recycled, or reused. Generally, the further downstream we go, the greater the potential flexibility that actors have to change the technology (Mulder & Knot 2001). At all stages, a number of context-specific factors mediate environmental and health risks and how actual impacts occur. These factors include local infrastructure and energy resources, access to specific technologies, the enforcement of government regulations, and actions by company executives, workers, government officials, advocacy groups, and consumers. Moreover, what happens at one stage can affect other stages profoundly. For example, activists may persuade a toy manufacturer to mandate the removal of a toxic additive, which feeds back upstream into the decisions of PVC manufacturers. We focus on the toy industry to examine the agents of change who have the capability to reduce harms created in the global PVC chain. PVC is widely used in children’s toys because of its durability, low cost, and ability to be molded (Tickner 1999). In the 1950s, the toy industry introduced a number of PVC-based products that became consumer icons: Mr. Potato Head (1952), Lego (1955), and Barbie (1959) (Meikle 1995). Injection molding and PVC offered a cheaper manufacturing route than more traditional materials. Prior to PVC, the toy company Mattel fabricated its commercial doll-heads with porcelain and doll-bodies with leather. To replace this design, Seymour Adler, the head plastics engineer of the Mattel Barbie product team, chose a soft PVC formulation to give the doll more physical detail, such as crevices between Barbie’s fingers and toes, and to strengthen its ability to withstand child play (Lord 2004). Today, Barbie is made with diverse plastic components: its arms are ethylene-vinyl acetate (EVA), its torso is acrylonitrile-butadiene-styrene (ABS), and its bend-leg armature has polypropylene. Its outer legs are still made of PVC, albeit with a different formula requiring less plasticizer. Since the late 1990s, advocacy groups and concerned scientists have criticized the toy industry for its use of PVC. Toy manufacturers from Hasbro to Lego, along with large toy retailers including Toys’R’Us, Walmart, and Target, have faced intensifying calls to make toys safer for young children, who are particularly susceptible to carcinogenic and endocrine-disrupting substances during their physical and cognitive growth (Iles 2007). In response, some toy companies are trying to remove PVC from their products in favor of safer plastics, or to reformulate PVC with non-toxic additives. In turn, PVC manufacturers continue to face long-running pressures from workers and fence-line communities to reduce their exposure to harmful vinyl chloride monomers and to hazardous chemicals like mercury used in producing chlorine (Thornton 2000). Chemists and executives in these firms can try to switch to alternative feedstock to make PVC-like plastics, or to redesign production processes early in PVC’s life cycle. Such decisions can ripple downstream but may be difficult to make, given existing industry structures. We will analyze the ethical arguments that chemists, engineers and business managers may consider when deciding whether and how to use green chemistry solutions. To do this, we concentrate on the PVC production and downstream product design/production lifecycle stages. (The end-of-life stage also poses many concerns that green chemistry could address, notably poor PVC recycling rates and high resistance to degradation in landfills.) 4.1 Raw materials and PVC manufacturingImpactsPVC exemplifies the complex web of material inputs and manufacturing steps that characterize the chemical industry. Commonly, a plastic is made by polymerizing a monomer that is in turn produced from a number of intermediate chemicals, all of which have their own feedstock sources. The initial choice of feedstock and basic chemicals can cause a cascade of multiple environmental and health damages along the lifecycle chain. For PVC, two major feedstock concerns exist: chlorine and petrochemicals. Moreover, the choice of intermediate chemicals and processing pathways can create further deleterious impacts. In this case, turning vinyl chloride monomer into PVC is associated with high cancer risks for factory workers. In terms of toxic effects alone, PVC use raises grave concerns. In both the acetylene and ethylene routes, VCM producers must use hydrogen chloride, which is formed by reacting chlorine gas and hydrogen gas at temperatures above 250° C (Wilkes et al. 2005). Chemically, elemental chlorine and hydrogen chloride are toxic at high concentrations; chlorine is also vigorously reactive. They are logical candidates for replacement by safer alternatives. Moreover, manufacturing both chlorine and acetylene uses mercury, an extremely dangerous substance. Traditionally, chlorine is produced by electrolysing brine salt in chlor-alkali factories. Not only does this electrolysis consume copious energy, it also depends on the use of mercury as a cathode (Wilkes et al. 2005). In the last century, chlor-alkali production has been second only to the fossil fuel industry in mercury releases to the environment. Humans can ingest mercury by eating fish and wildlife contaminated by industrial emissions. Even in minute amounts, mercury can cause numerous health effects on human bodies, including impaired neurological development in children (Thornton 2000). Many mercury-cell factories have been decommissioned (in favor of membrane and diaphragam technologies) due to concerns about mercury. Between 2005 and 2010 alone, global mercury-cell capacity decreased by about 30 percent (Global Mercury Partnership 2012). Still, over 100 mercury-cell factories continue operating worldwide. Further downstream, the acetylene route to make VCM uses a mercury catalyst-mediated addition of HCl to acetylene. In 1952, the Chisso Chemical Company began manufacturing acetylene at its factory on Minamata Bay in Japan. It discharged mercury sulphate effluents into the bay, eventually resulting in over 20,000 local inhabitants suffering or even dying from neurological diseases and birth defects (Hylander & Goodsite 2006). Other acetylene factories have been associated with similar health damages for neighboring communities. The ethylene route for manufacturing VCM does not involve mercury and is less energy intensive. However, ethylene production emits extremely hazardous organochlorine by-products generated during the oxychlorination process from the synthesis of EDC to make VCM (Thornton 2000). These substances include chlorinated dioxins, chlorinated furans, and polychlorinated biphenyls (PCBs). They can travel into the environment through various pathways, such as incinerating PVC waste, recycling vinyl-containing metal products, and accidental fires in buildings, warehouses, or landfills. These organochlorine substances often display persistent bioaccumulative toxicity: they strongly resist degradation, accumulate in fatty tissues in humans and wildlife, and can be widely dispersed by air and water. Dioxins in particular are carcinogenic, cause reproductive and developmental problems, and damage the immune system. Waste from ethylene-based VCM facilities in the US and Europe have some of the highest known dioxin concentrations (Thornton 2000). Further downstream, a larger number of companies are involved in polymerizing VCM into PVC and formulating hard and soft varieties of PVC for specific applications. They include firms like Shin Etsu Group, Formosa Plastics Corporation, Solvay, Ineos and Oxy Vinyls. Some of this PVC may require further processing before sale to distributors, retailers, or end-use consumers. The health risks of PVC have been known – and deliberately obscured – for at least 70 years (Soffritti et al. 2013). From 1938, European manufacturers observed that VCM was toxic in animals at low levels of exposure but kept these studies secret (Markowitz & Rosner 2002). By the 1950s, firms such as Dow Chemical and B.F. Goodrich were monitoring their workers through collecting urine and blood samples. They discovered that VCM exposure led to an increased probability of developing liver angiosarcoma and to statistically significant excesses of brain cancer and neurological effects not only in workers but in their families. Seeking to weaken calls for regulatory action, these firms publicly claimed that PVC products were benign. Starting in the 1970s, though, US regulators discovered the hidden data and imposed strict workplace exposure standards that forced US factories to steam-strip PVC in closed polmerization vessels (Soffritti et al. 2013). This step reduced VCM levels in PVC resins by 99%. Unfortunately, many older factories continue to operate globally. SolutionsHow might green chemistry solutions be used to address the raw material and initial manufacturing impacts of PVC? Chemists and business executives could contemplate actions like substituting new membrane technologies instead of brine electrolysis; replacing hazardous catalysts like mercury-based ones with safer catalysts; abandoning acetylene in favor of ethylene; or using different feedstock such as biomass to make ethylene while reducing the use of fossil fuels. Alternatively, companies can stop using PVC altogether as a plastic; they could use safer and sustainable plastics instead, thereby eliminating chlorine altogether. Here we consider two examples of green chemistry solutions: catalysts and biomass feedstock. A core green chemistry principle is to use catalysts rather than traditional stoichiometric reactions because these can accelerate reactions, increase efficiency of conversion, and reduce energy inputs with lower temperatures and pressures needed. In many cases, existing reactions may need to be redesigned to accommodate catalysts. In making acetylene, a catalyst is already used, but it may not be as efficient as modern catalysts. Many catalysts, like mercury-based ones, are toxic (mercury catalysts are short-lived because of their rapid loss of mercury, Johnston et al. 2015). The principle also suggests the substitution of toxic and polluting catalysts with benign ones. This green chemistry technique has been particularly popular in industry because it is a drop-in solution that does not call for extensive change yet can lead to substantial efficiency and economic cost gains. Interestingly, a new international mercury treaty requires VCM factories to switch to a mercury-free catalyst by 2017, assuming there is an economically available alternative. This has incentivized academic and industry researchers to search for new catalysts in anticipation of the treaty’s implementation (Zhang et al. 2011). One viable alternative is a gold nanoparticle catalyst technology that researchers at the Johnson Matthey Company (a leading catalyst maker) are commercializing now (Liu et al. 2014). This idea has already been around for 30-plus years. In 1985, Cardiff University chemist Graham Hutchings discovered that gold could catalyze the hydrochlorination of acetylene to VCM; later, he found that gold performed far better than mercury (Perks 2010). But only in the past few years has a chemical firm chosen to develop this idea into an actual technology. One reason is that China is one of the few countries where the acetylene route is still used intensively, and where mercury pollution is a pressing concern. Johnson Matthey identified a potential new market in China for a green acetylene catalyst and thus invested in its R&D for eight years to reduce the use of aqua regia in making the catalyst and to increase longevity and turnover. Its scientists screened many cationic gold-carbon complexes to identify the ones that performed the best in producing acetylene. A small pilot plant was installed at a Chinese PVC manufacturer, resulting in 99% selectivity, lower energy use, and less toxicity (Johnston et al. 2015). The manufacturer has now built a full plant, implying it thinks the technology is economical, while Johnson Matthey has located a new gold catalyst factory in China. Replacing mercury catalysts, however, cannot address the problems that choice of feedstock and intermediate chemicals can create. This is a limited process change. Moreover, gold nanoparticles may also be toxic to workers: the scientific evidence to date is contentious but suggests some potential harmfulness to cells (Frattoni et al. 2015). Another green chemistry principle that VCM and PVC manufacturers may consider to reduce the health and environmental costs of using fossil fuels is to use renewable feedstocks or inputs. They can choose to use biomass feedstocks – such as agricultural crops, grasses, and crop harvesting waste – in making PVC. In general, biomass feedstocks can yield dramatic decreases in greenhouse gas emissions, compared to petrochemical feedstock like naptha. These feedstock can also help avoid the larger social, geopolitical, and environmental impacts of extracting fossil fuels. Bio-plastics are a small but fast-growing segment of the polymer industry, with significant progress being made in the past decade to develop bio-based pathways to supply major existing chemicals or novel plastics (Iles & Martin 2013). Examples include manufacturing polyethylene from sugarcane, polylactic acid (PLA) from corn, and polyethylene terephthalate (PET) from corn. This work draws on the long history of lipid and carbohydrate chemistry to enable the use of materials such as fatty/oily or woody plant materials. Fermentation is a traditional bio-conversion technology that is now widely used to make bioplastic precursors. In some cases, microbes (including yeasts and bacteria) are genetically modified to preferentially metabolically convert raw materials in ways that naturally occurring species could not perform. In other cases, naturally available enzymes and microbes have been identified to perform such transformations. Regarding PVC, significant industry research has been underway for some time to develop a biobased version of PVC, using biomass to make ethanol (a biofuel) that can then be converted to ethylene. Bioethanol is produced by liquefying and fermenting sugary materials (primarily corn and sugarcane but potentially other crops like sugar beet), and distilling ethanol from the resulting mixture. It is also possible to obtain ethanol from hydrolyzing starch into glucose, or from using cellulosic technologies with corn stover (DuPont has opened a commercial scale plant in Iowa) or sugarcane bagasse (currently only at the pilot stage). From the 1950s, the Brazilian chemical producer Salgema used an old technology to make 100,000 tons of PVC from sugarcane ethanol annually, before going out of business due to competition from petrochemicals (Grushkin 2011). More recently, in the 2000s, the Brazilian subsidiary of Solvay, a Belgian-based chemical producer, began experimenting with new, more efficient techniques for making PVC from sugarcane ethanol. Plans were underway in the early 2010s for building a small factory but have not yet been realized, because of technical challenges and the difficulties of competing economically with petrochemical PVC. By contrast, Braskem, the leading Brazilian chemical firm, has successfully made ethylene from sugarcane ethanol for processing into polyethylene for some years already. The firm could extend this process to manufacturing PVC but has prioritized its polyethylene market. At present, bio-based chemicals tend to be more expensive than their oil-based counterparts, so they must either have superior technical attributes or be capable of attracting sustainability price premiums, to justify their manufacture (Iles & Martin 2013). This does not yet seem to be the case for bio-PVC, which is likely to be more costly than oil-based PVC. 4.2 Consumer Product Use and Phthalates ExposureImpactsFurther downstream, PVC suppliers and product manufacturers work together to develop formulations of the plastic for use in goods. Their choices regarding what to include in PVC can lead to consumers and factory workers being exposed to toxic substances. Suppliers mix additives with PVC resins to achieve performance characteristics according to customer specifications (Wilkes et al. 2005). Hundreds of chemical additives are now used to give PVC products various combinations of characteristics; they include stabilizers, lubricants, plasticizers, impact-modifiers, fillers, flame retardants, and reinforcing agents. Without these additives, PVC would be less durable and flexible, limiting its market. As PVC products are used, they can leach, flake or outgas plasticizers and stabilizers. In 2006, 5.8 million metric tons of plasticizers were used, far dwarfing stabilizers at 670,000 tons, let alone other additives (Makarian 2006). We will therefore focus on plasticizers as an example.[3] In this life cycle stage, manufacturers theoretically have much greater capability to change between different formulations, such as switching from phthalate plasticizers and lead stabilizers to safer alternatives. Yet removing the chemicals is complicated because of their role in making ubiquitous PVC use feasible. Plasticizers are a particularly important additive because they transform brittle PVC into a soft, malleable yet strong and stable plastic. Plasticizers and PVC resins are heated and mixed until they dissolve into one another. The plasticized material is molded into the product and cooled. The PVC-plasticizer relationship is unique because few polymers can retain high concentrations of plasticizers. PVC, however, responds favorably to plasticizers, which explains why PVC accounts for between 80-90% of all plasticizer consumption (Wilkes et al. 2005). Around 70 plasticizers are on the market. Product designers and manufacturers choose from diverse plasticizer options depending how soft, flexible, or heat-resistant the final product should be. The type and amount of plasticizer varies depending on the application. For example, PVC pipes require little to no plasticizers. By contrast, plasticizers can constitute 30% of the weight of PVC medical products (e.g. blood bags) and up to 80% of PVC recreational equipment (fishing tackle, children’s toys and bouncy balls). Phthalates are a popular plasticizer family that is compatible with PVC-based product and personal care products. Phthalates are typically formed by reacting a phthalic acid with an alcohol. Compared to other plasticizers, they are a general-purpose plasticizer that offers performance advantages not found in more specialized plasticizer families. These include strong solvency, low volatility, low diffusion, and flame resistance (Wilkes et al. 2005). Three phthalates account for about 75% of all plasticizers used in PVC: diisononyl phthalate (DINP), diisodecyl phthalate (DIDP) and di-2-ethylhexyl phthalate (DEHP). For decades, phthalates were thought by industry and regulators to be safe substances because they did not reveal any cancer-causing properties. It was not until the 1990s that scientific research began documenting that phthalates are potential endocrine-disrupting substances. Phthalates can interfere with the hormonal system, causing developmental and reproductive problems in both humans and animals. Numerous toxicological studies have now shown that phthalates can affect the growth of laboratory animals. A study of premature breast development in girls in Puerto Rico showed significantly higher levels of DEHP among girls with premature breast development compared with girls developing normally (Raloff 2000). Many toxicologists now agree that at least several commonly used phthalates pose substantial health risks to humans. Most phthalates, however, still have not been scientifically proven to be harmful even though they are suspected of being endocrine disrupters. PVC releases phthalates throughout its lifecycle, especially when it becomes overheated, agitated, or is stored too long and begins to breakdown. The larger population can be widely exposed to phthalates through ingestion, inhalation, and skin absorption. Phthalates can also cross the human placenta during pregnancy, thus affecting foetuses. Human biomonitoring surveys, such as those of the Centers of Disease Control in the US, suggest that virtually all Americans and Europeans contain phthalates in their blood and urine. Environmental health NGOs have used the CDC surveys to show that young children have particularly high levels of phthalates (Iles 2007). They attribute this disproportionate exposure to the fact that children play with soft PVC toys and put them into their mouths. Phthalate exposure from PVC toys is especially concerning because children are more vulnerable due to their smaller bodies and critical periods of physical and cognitive development. In response to this emerging new science, during the mid-2000s, a number of governments began eliminating a few commonly used phthalates from use in cosmetics and soft toys targeted at children (Iles 2007). Starting in 2004, the European Union took precautionary action and imposed short-term bans on three phthalates that were later permanently extended through the 2007 REACH chemicals regulation. Since 2008, the US federal government has also restricted these phthalates nationwide as part of a law reforming the Consumer Product Safety Commission. Chemical firms and many toy manufacturers have denied either that phthalates pose risks or have claimed that children’s products contain safe levels of phthalate plasticizers (Iles 2007). Nonetheless, a number of retailers and toy companies have announced they will phase out PVC altogether, or will eliminate phthalates in their products. Such corporate decisions are driven not just by regulatory bans but by consumer demands, NGO campaigns, and retailers’ own fears about legal liability, loss of market share, and damage to public reputation. SolutionsHow might green chemistry solutions be used to address the impacts of PVC product design choices? A fundamental green chemistry principle is to design safer chemicals. Toy companies and their designers can specify the chemicals they want to use in products, and can work with their suppliers to come up with new formulations or feedstock. Accordingly, one key green chemistry solution designers can use is to reformulate PVC so as to remove its toxic additives in favor of safe additives. They can also choose safer and more sustainable plastics other than PVC. As part of the toy industry’s growing response to criticism of PVC and phthalates, chemists and PVC makers have begun researching plasticizer alternatives. A minimal approach by numerous manufacturers is to ‘drop in’ other phthalates with safer health but comparable performance/cost profiles. One example is DINP (diisononyl phthalate). Because scientists disagree strongly over whether DINP is safe for children, the Consumer Product Safety Commission has declined to regulate DINP to date, arguing that few children are at risk. Some tests have shown that ingesting DINP causes kidney and liver damage in animals (Ackley 2000), which has led to the European Union restricting its use. In 2015, California listed DINP as a carcinogen on its Proposition 65 list. This means that retailers and manufacturers must label DINP-containing products with toxicity warnings, and hence now face greater regulatory pressure to abandon DINP. A more demanding approach is to introduce different substances as plasticizers and remove phthalates altogether. These substances include citrates, sebacates, adipates, and phosphates (Tickner 2011). For example, acetyl tributyl citrate, bis(2-ethylhjexyl)-1,4-benzenedicarboxylate (Eastman 168), and dioctyl terephthalate (DOTP) are being used in some PVC toys. Some companies are exploring biobased alternatives to plasticizers. Metabolix, a renewable chemicals firm, has been developing polyhydroxyalkanoate (PHA) co-polymers that can substitute for some (not all) of the (fossil fuel-derived) phthalates used in PVC. Such co-polymers can impart greater durability and flexibility to PVC. PHA is made through bio-fermentation of sugars, where water-insoluble inert co-polymer builds up inside microbes that are pulped into a broth for extraction. Toxicological testing currently suggests that PHAs are non-toxic to humans. Similarly, Dow Chemical has introduced ‘BioVinyl’, which is a phthalate free plasticizer made from ill-defined ‘plant by-products’ and which promises a 41% decrease in greenhouse gas emissions compared to fossil fuel plasticizers. Unfortunately, for most of these phthalate alternatives, toxicological and environmental safety data does not yet exist (Tickner 2011). Companies were not obliged under the old Toxic Substances Control Act (the major US chemical regulation) to carry out studies prior to marketing them. Such alternatives can still leach out of products. For DOTP, toxicity testing suggests that it is slightly irritating to eyes and may cause dermatitis; it is likely to biodegrade readily and should not build up in ecological food webs. By contrast, Eastman 168 lacks any health and environmental data. If companies choose phthalate-free alternatives, they risk creating new health hazards that may take time to manifest and be recognized. The most radical approach is to eliminate PVC and use another plastic (or even different materials like metal or wood). Researchers looked at a set of 55 plastics on the market and concluded that PVC is easily the worst plastic because of its unique combination of chlorine, VCM, and additive toxicity (Lithner et al. 2011). Many other plastics would be significantly safer, if not necessarily sustainable. Starting in the late 1990s, NGO and academic analysts have repeatedly pointed out that toy manufacturers do have a wide variety of plastics options to choose from if they desire to jettison PVC. In 2011, scientist Joel Ticker surveyed these options and reported that, for example, high-density polyethylene (HDPE) could be substituted in, with relatively few effects, primarily the use of flammable chemicals in making it. While HDPE is recyclable, it cannot be biodegraded or composted. Nonetheless, most of the petrochemical-based plastics still posed serious toxicity and ecological concerns (Tickner 2011). At present, most potential biobased plastics are still not ready for use, and the main safer possibilities already on the market – PHAs and thermoplastic starch – demonstrate several problems. While PHA itself is viewed as non-toxic, its production currently requires the use of several highly toxic solvents. A number of toy makers – like Mattel, Hasbro, and Lego – are already switching to other plastics from PVC. To help, manufacturers could put pressure on plastics manufacturers to further innovate in PVC substitutes that match PVC’s performance attributes. For example, Lego has recently announced a $150 million program to support R&D into sustainable plastics. Chemists can work on improving the environmental performance of biobased plastics by tinkering with reaction pathways, replacing toxic inputs and solvents, and making reactions solvent-free. 5. Integrating Ethics into Green Chemistry Decision-MakingGreen chemistry offers diverse ways to help overcome technological lock-ins more generally. As we will see, it is more challenging to loosen up the historically rigid PVC production chain than for many other chemicals. Nonetheless, taking a green chemistry lens can enable managers, chemists, and designers to think differently about seemingly long-stabilized technologies, because green chemistry offers concrete solutions for their environmental problems while potentially improving performance. When companies, chemists, and engineers consider if they should pursue green chemistry solutions, they frequently conduct analysis – whether informal or formal – within a number of dimensions. These include the degree of change to existing production technologies and supply chains; perceived risk to the company vis-à-vis the magnitude and impacts of ecological and human health risks; and the economics and commercial benefits of installing a solution. Even if the precautionary principle is at the core of green chemistry, there can be utilitarian and deontological flavors, leading to more malleable or more absolute outcomes. The technical and economic considerations may be construed rather differently, according to the ethical stance that an actor takes. We can glimpse how ethical arguments might be made through considering the green chemistry choices that four types of companies and their various employees must make when deciding whether and how to act on PVC risks. Chlorine producers face the most challenging choice of all. Chlorine is clearly a highly harmful substance in both its gas and vinyl monomer forms. Should firms stop making chlorine and move into another chemical sector? In other words, should they abandon their core business? Because many chlorinated substances have been banned (e.g., DDT, PCBs) and chlorine is no longer permitted for use in many applications including refrigerants, solvents, and pulp bleaching, PVC has become an increasingly important market to maintain. As one chlorine producer stated: "Flexibility is no option for our company. The firm is captured in the structure of the molecules" (Mulder & Knot 2001, p. 341). Unless chlorine firms strongly believe in the ethical need to avoid harmful health effects, and are willing to change their business and market altogether, they are unlikely to do anything about chlorine itself. (Conversely, downstream consumer product makers can choose to relinquish chlorine much more readily, which may lead to a dwindling PVC market in years to come.) Further downstream, companies supplying monomer materials to PVC manufacturers must decide whether they want to use acetylene or ethylene as their input. Acetylene still remains a key route in some countries heavily reliant on cheap coal. Producers still using coal must decide whether to make the acetylene route safer by tweaking its design and technologies. One solution, as we saw, is to replace mercury catalysts with gold nanoparticles. Catalysts are a popular green chemistry solution precisely because they can often be dropped into existing processes and businesses. Business managers may see them as posing relatively low risk to a company in terms of destabilizing production, upsetting customers, and losing market share. Moreover, using catalysts can offer substantial efficiency and financial benefits. Changes that do not markedly alter a product design are more favorably regarded. In contrast, green chemistry solutions calling for extensive change may evoke trepidation. The precautionary principle may help justify more radical changes, or may make otherwise costly and disruptive changes worthwhile. In this case, the extreme hazards of mercury are robustly known – they are not uncertain or remote, a large number of people are being harmed, and the costs and technological changes are fairly modest. Most policy-makers and analysts, especially in the West, would agree that it is not even necessary to invoke the precautionary principle to justify eliminating mercury outright. Even an utilitarian accounting would likely support this outcome. Nonetheless, the industry calculations may differ somewhat in China and other countries still relying on acetylene, so that strong ethical arguments may be needed to change the status quo. Over the past 35 years, for example, the Chinese government has encouraged extraordinarily rapid industrialization without regard for population health. Hundreds of state-owned coal-fired power stations and many thousands of factories have been built in China, leading to horrendous air pollution and steep rises in cancer and respiratory disease rates. Development has been culturally prioritized ahead of public health. The acetylene route is associated with an industry cost structure based on lowest cost production. Producers may thus be less willing to modify their technologies because they face the unpredictable risks of deploying gold nanoparticles and possibly partnering with an unfamiliar, foreign catalyst supplier. Chemists at Johnson Matthey (developer of the gold alternative) argue that removing mercury from the acetylene route will enhance worker and resident health (Johnston et al. 2015). Yet managers and chemists in the Chinese firms may or may not agree that green chemistry is morally necessary to prevent a clearly damaging risk even if it causes their firm hefty expenses. They may assert that the health benefits of using nanoparticles are too diluted in the larger air pollution problem to matter. Or, they may come to believe that they do have the agency to help improve environmental health, perhaps because there is now a broad deontological duty on all manufacturers to alleviate the heavy burden of their people in whatever ways they can. As residents learn more about the costs of the pollution they are being exposed to, they are beginning to mobilize community protests against chemical factories, which may support this ethical view. In turn, PVC manufacturers increasingly must decide whether to source ethylene from petrochemicals, or from biomass materials. Many companies have long since switched to ethylene from acetylene, largely because of ease of use of oil and natural gas. It would be potentially a green chemistry solution to take up a different route that offers less toxicity, fewer energy and water inputs, and fewer waste by-products. The trouble is that petrochemical-based ethylene is not much better, because of its high, well-known climate change and toxicity impacts. Bio-based ethylene might alleviate these impacts, might release many lock-ins, and is already a proven technology as far as Braskem’s green polyethylene goes. Researchers, however, have carried out lifecycle assessments that suggest that biobased PVC could have mixed benefits (Alvarenga et al. 2013). While the chemical offers dramatically lower greenhouse gas emissions and consumption of non-renewable resources, it can manifest higher impacts (compared to petrochemical-based PVC) in water use, biodiversity, land use, eco-toxicity, and other areas. Importantly, biobased chemicals can contain substantial toxicity risks, notably from the petrochemical solvents used in their production (Álvarez-Chávez et al. 2012). To some extent, all these adverse effects can be avoided by corporate managers insisting on developing green chemistry designs that foresee them – such as by eliminating the solvents. Because most of these impacts are not yet well-understood and are uncertain, such action would be necessarily precautionary in nature. Whether or not biobased PVC is actually superior to petrochemical PVC mostly depends on how, and where, biomass is produced. Here, firms have many considerations to evaluate, such as upstream agricultural impacts (Iles & Martin 2013). Corn is a common bioplastic feedstock. Yet corn grown in the US is notorious for its vast environmental effects, including fertilizer run-off that causes an ocean ‘dead zone’ in the Gulf of Mexico, large pesticide use coupled with GM crop use, and loss of biodiversity due to monoculture farm fields. By contrast, sugarcane grown in Brazil can be less ecologically damaging – unless it causes, directly or indirectly, the conversion of high-carbon containing rainforest and cerrado lands. In both cases, the method of farming matters greatly: if industrial agriculture technologies are used, there will still be intensive use of fossil fuels for energy and chemical inputs. The next generation of biobased materials – cellulosic biomass such as grasses – is likely to perform better than food crops, but could still be produced on industrial plantations. But if diversified, agroecological farming methods are used to grow sugarcane or, better, grasses, lower environmental impacts may result (Jordan et al. 2016). In other words, chemists and managers will need to learn more about previously overlooked areas if they are to appreciate the full implications of their choices. They will need to develop a sense of not only chemical but agricultural ethics in order to better grasp the range of uncertainties that underline biobased feedstock, and thus see whether they must take precautionary action in terms of how this feedstock is produced. If chemical firms still stick with PVC, their managers must decide whether and how to modify their formulations to address additive risks. Phthalates are only one of the many additives that can be found in PVC. Should PVC makers jettison phathalates altogther in favor of safer alternatives? If so, which alternatives should they consider? Here, the precautionary principle has been intensely debated among firms. While evidence that some phthalates are endocrine disrupters and carcinogens is growing, sizable uncertainty about their effects remains. This is all the more true of most phthalate substitutes: many firms have been developing substitutes on the basis that these could create new markets as phthalates dwindle. Yet little health and ecological data attests to their safety, so that a substitute can be introduced only to be later proven to be harmful. Many firms seem to be adopting an utilitarian precaution: the substitutes still benefit more people in guarding against a known phthalate risk, while the economic and societal costs of eliminating PVC are still too large to accept. Nonetheless, for many substitutes, there is a sliver of scientific evidence of risk. Biobased additives also raise similar moral questions as for biobased ethylene. In response, some chemists may argue that the precautionary principle should be implemented at its most stringent. Even though a substance has not yet been proven to be injurious, enough reliable scientific evidence exists to justify discarding a chemical. Only those substances that have passed exhaustive testing should be accepted onto the market. There appears to be widening agreement among chemists regarding phthalates – but not on which alternatives are tolerable. Finally, both toy manufacturers and retailers must confront their own ethical quandaries – but ones reflecting the consumer’s perspective. These firms may have the greatest capacity to change from PVC and its associated chemicals, in that they do not depend on PVC or chlorine for their business success. They are preoccupied with product functions, not with specific materials. Retailers and toy companies are directly exposed to consumer demands and buying choices. As seen in a recent NGO report ranking leading retailers on their performance in dealing with chemical risks (Safer Chemicals Campaign 2016), retailers are much more visible than most chemical firms. Thus, these firms may have a rather different calculus regarding whether and how to adopt green chemistry, because they are much more exposed to societal values regarding chemical risks. Toy companies can share the views of PVC makers in being reluctant to abandon PVC as an uniquely valuable plastic used in countless toys. But growing consumer and scientific concern about PVC additives can lead to managers and product developers making precautionary choices if they are not yet firm believers in endocrine disrupting risks. A large number of firms across many consumer product and medical equipment sectors are simply choosing to ‘go PVC-free’, preferring safer plastics. In their eyes, it is just too much trouble to try to ‘repair’ PVC, especially if it has high overall toxicity (due to chlorine and vinyl monomers). Likewise, retailers can tell their suppliers they will not stock PVC toys – sending a powerful market signal upstream to toy and PVC manufacturers. Toy makers can also request safer additives and truly sustainable bio-based ethylene to make PVC less damaging, if they are willing to tolerate its upstream production impacts. The choices that companies make here will be driven by how far they believe they must adopt the precautionary principle, and how far they want to reach back into the production chain. 6. ConclusionBy studying the PVC case, you can learn several important lessons for use in your future careers. PVC underscores the profound ethical challenges of trying to change a plastic manufacturing system that has become structurally inert over many decades. In this case, how should companies deal with the contradictions of using basic chemicals to make numerous intermediate and final products, while also causing numerous environmental, social, and health problems? As we have seen, a growing number of firms and scientists are experimenting with green chemistry for business, regulatory, and ethical reasons (Iles 2007). In offering powerful new capabilities to create alternative pathways, green chemistry profoundly challenges ‘brown chemistry’, or the petrochemical-based processes that generate vast quantities of waste and pollution along with poisonous chemicals (Woodhouse & Breyman 2005). Many academic green chemists say their profession helped build a world heavily polluted with toxics; they now want to heal this planet with safer, more sustainable chemicals. Because of its precautionary roots, green chemistry is itself an ethical vision of what chemicals could be. In Europe, California, and many other locations around the world, some policy-makers, industrial chemists, and business executives have joined green chemists in seeking innovations such as discovering safe solvents and making reactions more efficient (Mulvihill et al. 2011). Yet PVC illustrates why and how applications of the principles can be gnarly in practice. The complex PVC production chain means that a single solution is unlikely to succeed fully: multiple solutions may have to be imperfectly patched together. Some solutions may worsen toxicity risks while achieving greater atom efficiency, creating ethical dilemmas that may only be decided by prioritizing some impacts over others. Moreover, numerous companies – petrochemical firms, catalyst makers, specialty chemical manufacturers, plastic processors, consumer product companies, and retailers – are involved in making or using PVC. The ethical responsibility for acting may, then, be diffused across many industry actors that diverge markedly in their philosophies and capabilities. Most crucially, PVC represents an extreme example of technological and business lock-ins that inevitably color company decisions as to what can (or should) be done. Namely, without PVC, affordable chlorine could not be made; without chlorine, PVC could not be made. As a result, applying green chemistry in practice may not be straightforward. Companies can face difficult decisions regarding whether to use green chemistry solutions, how, and to what degree. As seen in the PVC case, green chemistry practice can have utilitarian and deontological flavors. Scientists and managers are now aware that PVC and its many constituents (from feedstock to additives) cause grave environmental and health problems. Some of these are well understood (like mercury) while others are still ill-known. There are strong reasons – both precautionary and proven – to intervene to change PVC production in various ways. But companies may argue that they cannot use green chemistry because it would perturb their existing operations, or because they are locked into particular technologies. Other firms may say that they will adopt green chemistry solutions only to the extent that these can readily be dropped in without financial cost or market disruption. Still other companies may be willing to make fundamental changes on the ground they are morally responsible for the resulting ecological impacts. They may also perceive that customers are demanding safer chemicals or that new markets could materialize. PVC is only one of many chemicals locked into production chains with major environmental and health impacts resulting. Once a chemical is put on the market, companies have historically had little incentive to review how it was designed and produced. Countless ‘older’ chemicals persist from the 1930s to 1960s period (often seen as the golden era of chemical innovation). Some examples are polystyrene, flame retardant chemicals, and perfluorinated chemicals, which have permeated consumer products, and whose designs reflect decades of congealed assumptions, negligible toxicological testing, and lax regulation. Green chemistry gives chemists another chance to re-think these legacy chemicals, this time for health and sustainability reasons. Through green chemistry, industry lock-ins can be dismantled. And in developing new materials, further lock-ins can be avoided with greater anticipation, using the precautionary principle. To do so, chemists will need to put ethical questions at the center of their work – and to resist the allure of succumbing to the inertia of ‘this is how things have always been done’. Notes[1] For explanation of risk assessment and its limitations in the context of US chemicals regulation, see Martin, Iles & Rosen 2016. [2] In many cases, the ‘real’ benefits of action may be large but be excluded through CBA’s selective omission of facets like performance in school and longer term employment prospects on the basis these cannot be measured, or given monetary value. [3] Historically, heat stabilizers frequently made use of lead and other heavy metals. Around 2006, the European PVC industry committed to phasing out lead stabilizers by 2015 in favor of mixed metal stabilizers (Makarian 2006). Further ReadingFor further investigation of green chemistry ideas, the following reference books are helpful: Lancaster 2016, Matlack 2010, and Clark & Macquarie 2008. These are leading textbooks or handbooks on green chemistry that are accessible at the undergraduate level. ReferencesAckerman, F.: 2008, Poisoned for Pennies: The Economics of Toxics and Precaution, Washington DC: Island Press. Ackley, D.: 2000, ‘Effects of Di-isononyl Phthalate, Di-2-ethylhexyl Phthalate, and Clofibrate in Cynomologus Monkeys’, Toxicological Sciences, 56, 181-188. Alvarenga, R.; Dewulf, J.; De Meester, S.; Wathelet, A.; Villers, J.; Thommeret, R. & Hruska, Z.: 2013, ‘Life cycle assessment of bioethanol-based PVC’, Biofuels, Bioproducts and Biorefining, 7 (4), 386-395. Álvarez-Chávez, C.; Edwards, S.; Moure-Eraso, R. & Geiser, K.: 2012, ‘Sustainability of bio-based plastics: general comparative analysis and recommendations for improvement’, Journal of Cleaner Production, 23 (1), 47-56. Anastas, P.T. & Warner, J.C.: 1998, Green Chemistry: Theory and Practice, New York: Oxford University Press. Anastas, P.T. & Williamson, T.C.: 1996, Green Chemistry: Designing Chemistry for the Environment, Washington, DC: American Chemical Society. Ashford, N.: 2007, ‘The legacy of the precautionary principle in US law: The rise of cost-benefit analysis and risk assessment as undermining factors in health, safety and environmental protection’, in: N. de Sadeleer (ed.), Implementing the Precautionary Principle: Approaches from the Nordic Countries, the EU and the United States, London: Earthscan. Chandler, A.: 2009, Shaping the Industrial Century: The Remarkable Story of the Evolution of the Modern Chemical and Pharmaceutical Industries, Cambridge, MA: Harvard University Press. Clark, J. & Macquarrie, D.: 2008, Handbook of Green Chemistry and Technology, New York: John Wiley & Sons. Collins, T.: 2001, ‘Toward sustainable chemistry’, Science, 291 (5501), 48-49. European Environmental Agency: 2001, Late lessons from early warnings: the precautionary principle 1896-2000 [available online at: http://www.eea.europa.eu/publications/environmental_issue_report_2001_22, accessed 3 February 2017. Faulkner, D.; Shen, L.; Vanessa, Y.; Johnson, D.; Hemingway, R.; Williams, R.; Arnold, J. & Vulpe, C.: 2017, ‘Tools for Green Molecular Design to Reduce Toxicological Risk’, in: Richardson, R, & Johnson, D. (eds.), Computational Systems Pharmacology and Toxicology, London: Royal Society of Chemistry, pp. 36-59. Fratoddi, I.; Venditti, I.; Cametti, C. & Russo, M.: 2015, ‘How toxic are gold nanoparticles? The state-of-the-art’, Nano Research, 8 (6), 1771-1799. Frickel, S.; Gibbon, S.; Howard, J.; Kempner, J.; Ottinger, G. & Hess, D.: 2010, ‘Undone science: social movement challenges to dominant scientific practice’, Science, Technology, and Human Values, 35 (4), 444-473. Geiser, K.: 2015, Chemicals without harm: policies for a sustainable world, Cambridge, MA: MIT Press. Global Mercury Partnership: 2012, Conversion from Mercury to Alternative Technology in the Chlor-Alkali Industry, Nairobi, Kenya: United Nations Environmental Programme. Grassie N.: 1966, ‘Degradation’, in: Encyclopedia of Polymer Science and Technology, New York: John Wiley, vol. 4. Grushkin, D.: 2011, ‘Breaking the mold’, Nature biotechnology, 29 (1), 16. ICIS Chemical Business: 2012; ‘China invents and reinvents coal to chemicals’ [available online at: http://www.icis.com/resources/news/2012/04/16/9549986/china-invents-and-reinvents-coal-to-chemicals/, accessed on 3 February 2017]. Hylander, L. & Goodsite, M.: 2006, ‘Environmental costs of mercury pollution’, Science of the Total Environment, 368 (1), 352-370. Iles, A.: 2007, ‘Identifying environmental health risks in consumer products: non-governmental organizations and civic epistemologies’, Public understanding of science, 16 (4), 371-391. Iles, A.: 2008, ‘Shifting to green chemistry: the need for innovations in sustainability marketing’, Business Strategy and the Environment, 17 (8), 524-535. Iles, A. & Martin, A.: 2013, ‘Expanding bioplastics production: sustainable business innovation in the chemical industry’, Journal of Cleaner Production, 45, 38-49. Knot, J.; Van den Ende, J. & Vergragt, P.: 2001, ‘Flexibility strategies for sustainable technology development’, Technovation, 21 (6), 335-343. Johnston, P.; Carthey, N. & Hutchings, G.: 2015, ‘Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination’, Journal of the American Chemical Society, 137 (46), 14548-14557. Jordan, N.R.; Dorn, K.; Runck, B.; Ewing, P.; Williams, A. & Anderson, K.A.; 2016, ‘Sustainable commercialization of new crops for the agricultural bioeconomy’, Elementa: Science of the Anthropocene, 4, 81. Liu, X.; He, L.; Liu, Y. & Cao, Y.: 2013, ‘Supported gold catalysis: from small molecule activation to green chemical synthesis’, Accounts of chemical research, 47 (3), 793-804. Lancaster, M.: 2016, Green Chemistry: An Introductory Text, 3rd edition, London: Royal Society of Chemistry. Lithner, D.; Larsson, Å. & Dave, G.: 2011, ‘Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition’, Science of the Total Environment, 409 (18), 3309-3324. Lord, M.: 2004, Forever Barbie: The unauthorized biography of a real doll, New York: Bloomsbury. Markarian, J.: 2007, ‘PVC additives: What lies ahead?’, Plastics, Additives and Compounding, 9 (6), 22-25. Markowitz, G. & Rosner, D.: 2002, Deceit and denial: The deadly politics of industrial pollution, Berkeley: University of California Press. Martin, A.; Iles, A. & Rosen, C.: 2016, ‘Applying Utilitarianism and Deontology in Managing Bisphenol-A Risks in the United States’, HYLE: International Journal for Philosophy of Chemistry, 22, 79-103. Matlack, A.: 2010, Introduction to green chemistry, Boca Raton, FL: CRC Press. Meikle, J.: 1995, American plastic: a cultural history, New Brunswick, NJ: Rutgers University Press. Mulder, K. & Knot, M.: 2001, ‘PVC plastic: a history of systems development and entrenchment’, Technology in Society, 23 (2), 265-286. Mulvihill, M.; Beach, E.; Zimmerman, J. & Anastas, P.: 2011, ‘Green chemistry and green engineering: a framework for sustainable technology development’, Annual review of environment and resources, 36, 271-293. Perks, B.: 2010, ‘Gold Fever’, Chemical World, September, 48-50. Raffensperger, C. & Tickner. J.: 1999, Protecting public health and the environment: implementing the precautionary principle, Washington DC: Island Press. Raloff, J.: 2000, ‘Girls May Face Risks from Phthalates’, Science News, September 9, 165. Soffritti, M.; Sass, J.; Castleman, B. & Gee, D.: 2013, ‘Vinyl chloride: a saga of secrecy’, in: EEA (eds.), Late Lessons from Early Warnings: The Precautionary Principle, 2nd edition, Copenhagen: European Environmental Agency, pp. 179-202. Spitz, P.H.: 1988, Petrochemicals: The Rise of an Industry, New York: Wiley. Thornton, J.: 2000, Pandora’s Poison: Chlorine, Health and a New Environmental Strategy, Cambridge, MA, MIT Press. Tickner, J.; 1999; A Review of the Availability of Plastic Substitutes for Soft PVC in Toys, Greenpeace International. Tickner, J.; Raffensperger, C. & Myers, N.: 1999, The Precautionary Principle in Action: A Handbook, Windsor, ND: Science and Environmental Health Network. Tickner, J. & Geiser, K.: 2004, ‘The precautionary principle stimulus for solutions-and alternatives-based environmental policy’, Environmental Impact Assessment Review, 24 (7), 801-824. Tickner, J.: 2011, ‘Phthalates and Their Alternatives: Health and Environmental Concerns’, Lowell Center for Sustainable Production [available online at: https://www.sustainableproduction.org/downloads/PhthalateAlternatives-January2011.pdf, accessed 3 February 2017]. Wilkes, C.; Daniels, C. & Summers, J.: 2005, PVC Handbook, Cincinnati: Hanser. Wilson, M. & Schwarzman, M.; 2009, ‘Toward a new US chemicals policy: rebuilding the foundation to advance new science, green chemistry, and environmental health’, Environmental health perspectives, 117 (8), 1202. Woodhouse, E. & Breyman, S.: 2005, ‘Green chemistry as social movement?’, Science, Technology, & Human Values, 30 (2), 199-222. Vink, E.; Rabago, K.; Glassner, D. & Gruber, P.: 2003, ‘Applications of life cycle assessment to NatureWorks polylactide (PLA) production’, Polymer Degradation and stability, 80 (3), 403-419. Zhang, J.; Liu, N.; Li, W. & Dai, B.: 2011, ‘Progress on cleaner production of vinyl chloride monomers over non-mercury catalysts’, Frontiers of Chemical Science and Engineering, 5 (4), 514-520. Zion Market Research: 2016, ‘Global PVC Market Set for Rapid Growth, To Reach Around USD 78.90 Billion by 2021’ [available online at https://www.zionmarketresearch.com/news/global-pvc-market, accessed February 3, 2017. Alastair Iles (corresponding author): |