http://www.hyle.org
Copyright © 2016 by HYLE and Abigail Martin, Alastair Iles & Christine Rosen
Applying Utilitarianism and Deontology in Managing Bisphenol-A Risks in the United StatesAbigail Martin, Alastair Iles and Christine Rosen*
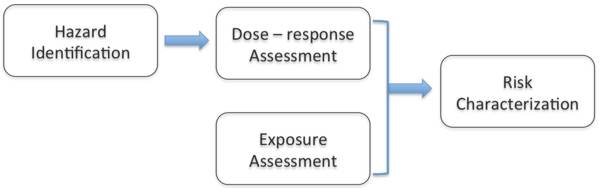
1. IntroductionBisphenol-A is a chemical used in consumer products such as baby bottles, reusable water bottles, and infant formula containers. The substance is found in many other products that require strong, clear glassy materials, such as electronics and food packaging. Some scientific studies have linked Bisphenol-A (BPA) to diabetes, thyroid disease, various cancers, and obesity, but experts disagree over whether BPA is causing harm through its ability to disrupt endocrinal functions. Many chemical and product manufacturers have defended BPA as safe despite concern about the risks of BPA from activists, consumers, and some scientists and researchers. Some companies have voluntarily replaced their products with BPA-free versions. Governments appear to be similarly torn: In Europe and Japan, government regulators consider BPA safe at current exposure levels, while experts advising the Canadian government concluded the opposite. The case of Bisphenol-A exemplifies societal debate over industrial chemicals in the 21st century. Over the past fifteen years, public concerns about chemicals embodied in consumer products have grown steadily. These chemicals can dissipate from products during their use and disposal, and can be absorbed or ingested into human bodies. In earlier decades, public concern and regulators largely focused on chemical risks created in the manufacturing phase, such as factory pollution and hazardous waste. Such risks are still significant. However, human exposure to consumer products occurs at a much greater order of magnitude: many more people are potentially affected once products leave the manufacturing plant. Today there is more attention to the health hazards of toxic chemicals in products, but regulators still struggle with the question of how to define toxicity. Traditional toxicology studies used by regulators to determine a chemical’s toxicity focus on whether a chemical is carcinogenic (cancer-causing), often overshadowing the question of whether a chemical is estrogenic, or capable of disrupting hormonal processes in the body. Although Bisphenol-A’s estrogenic properties have been known since 1938, its risks are still debated – with implications for the numerous chemicals used in consumer products that are suspected of being endocrine disruptors. The case of BPA exemplifies three key challenges in the chemical industry. First, tracing the growing concerns about human exposure to BPA shows how regulatory approaches can exacerbate the difficulty of dealing with the unforeseen risks of chemicals in consumer products. Second, the case raises the question of who should bear the responsibility – and the cost – of rectifying or preventing emerging chemical risks in consumer products. Third, BPA highlights the challenge of substituting a potentially hazardous chemical for a harmful substance in consumer products in the context of well-established global production chains and consumption patterns. Utilitarian and deontological ethical frameworks have influenced societal debates surrounding each of these three challenges. This article explores how these ethical frameworks raise moral dilemmas for the various actors involved – both those implicated in the production of harmful chemicals and those pursuing remedies: chemists designing molecules; managers devising business models for sustainable products; consumers making purchasing decisions; governments setting health and safety standards; groups pushing for clearer workplaces and products. Each of these actors have moral agency – the power to be morally accountable for one’s actions and their consequences. But who has moral agency to advance more sustainable outcomes for the public good? When these actors face a moral dilemma, what ethical perspectives determine what it means to ‘do the right thing’? In the next few sections, we review the history of BPA use in manufacturing products and track the changing science and perceptions of BPA toxicity risks, before turning to discuss the ethical dilemmas of key actors in the BPA production chain. While reading this background, you should reflect on what responses might be appropriate in a situation in which a chemical risk is not yet fully proven but the chemical is commercially lucrative. 2. A short history of Bisphenol-ABPA – also known as 2,2-bis-4-hydroxyphenyl – is a synthetic chemical found in numerous products, including automotive parts, water supply pipes, electronics, baby bottles, and other food containers. It is one of the highest production volume chemicals in the world. By the 1980s, the global production of BPA reached almost a million metric tons per year and has grown substantially since then (Fiege et al. 2012). According to industry reports, the global demand for BPA was over 6 million metric tons in 2013 – representing a market size of $US 13.87 billion (Grand View Research 2014). Manufacturing capacity was once concentrated in the US, Europe, and Japan, but has expanded to Asia as markets for BPA and consumer end-products made with BPA have become increasingly global. BPA gained commercial success in the polymer and plastics production with two main end markets: approximately 63% of BPA is used to build polycarbonate plastic resins and 27% goes into formulating epoxy resin monomers. For both polycarbonates and epoxy resins, BPA is an important building block with attributes that industry has found difficult to match with substitute chemicals (Ritter 2011). In the US, just five companies manufacture BPA: Bayer, Dow, Hexion Specialty Chemicals, SABIC Innovative Plastics (formerly GE Plastics), and Sunoco collectively generate approximately $US 6 billion in sales per year (Case 2009). BPA was first synthesized in 1891, but its commercial production did not begin until the early 1950s, after chemists created the first epoxy resins using BPA in the US and Europe (Vogel 2009). Epoxy resins are produced by transforming liquid polyethers into infusible solids through a special curing process that reacts epichlorohydrin with BPA. These resins are versatile chemicals that can be formulated to have a range of mechanical properties (from extreme flexibility to high strength and harness), chemical resistance, high adhesive properties, and high electrical resistance. Epoxy resins became extensively used throughout the manufacturing sector as protective coatings for metal equipment, piping, steel drums, and the interior of food cans. In 1957, chemists at Bayer and General Electric began developing another use for BPA as a monomer feedstock in plastics production (Vogel 2013). When polymerized with either carbonyl chloride or diphenyl carbonate, BPA forms a plastic called polycarbonate. Polycarbonate is hard, clear and nearly unbreakable. It is often used to replace glass in a variety of consumer products. The most common trade name for polycarbonate is Lexan. 3. Diverging Opinions on Whether BPA is ‘Safe’BPA is not only ubiquitous in everyday materials but is also prevalent in human bodies. Biomonitoring studies of the American population, for example, have consistently shown the widespread presence of BPA in urine, with slightly elevated levels in children, females, and lower-income populations (Calafat et al. 2008, Vandenberg et al. 2007). In its Fourth National Report on Human Exposure to Environmental Chemicals, the US Centers for Disease Control found detectable levels of BPA in 93 percent of urine samples from over 2,500 people, suggesting almost universal and continual exposure (Centers for Disease Control 2009). Although the amount of BPA found in human bodies is usually relatively low, experts strongly disagree about the levels at which exposure to BPA is harmful. Early toxicology studies in the 1970s found no observable carcinogenic effects in rodents given high doses of BPA for two years, in part because BPA metabolized rapidly in the animals’ bodies. Yet, in the last two decades, new scientific research has ignited regulatory controversies over BPA. A growing number of studies suggest that repeated small doses of BPA can disrupt the human endocrine system, especially during prenatal and post-natal development. Fetuses and infants thus have a heightened risk of developmental harm when exposed to BPA transmitted through the placenta or in breast milk, or through containers like baby bottles. Although environmental and public health advocacy organizations have called on governments worldwide to ban the use of BPA in food packaging, regulators diverge over whether there is enough evidence to justify controls. The challenge is how to decide what this level is, in a situation where scientifically credible studies can substantiate a cause-and-effect relationship between BPA and adverse health outcomes whereas other scientifically credible studies undermine this relationship. Facing apparently conflicting scientific evidence, regulators can reach different conclusions about whether BPA is safe, because of their differing interpretations of the data and because of their assumptions about which risks and harms matter. For example, within Europe, there is a splintering of views underway. The European Food Safety Authority believes that BPA is safe for use in food packages, while France has banned this particular application from January 2015 (Jacobsen 2015). In the US context, a federal system of government exists, in which federal, state, and local levels of government have their own jurisdictions (or areas over which they can wield legislative and executive powers). Most chemical regulation occurs at the federal level but states and cities can pass their own laws or bans, as long as the federal government has not displaced these with its own. Three federal regulatory agencies are typically involved in overseeing chemical risks: the Food and Drug Administration (FDA) for food; the Environmental Protection Agency (EPA) for toxic substances and pesticides; and the Consumer Safety Protection Commission (CSPC) which has acquired some jurisdiction over phthalates in toys and cosmetics. Each agency must implement federal laws that gives it specific powers, often founded on doing risk assessments to decide whether a chemical must be regulated. In other words, chemical risk regulation is divided between these agencies, leading to many gaps in oversight. In 1963, FDA approved BPA as "generally regarded as safe" for use in food additives (Turker 2012). Polycarbonate plastics and epoxy resins are regarded as food additives if they come into contact with food. This approval means that regulators have neglected BPA for many years, assuming that it remains safe. It is up to NGOs and citizens to petition FDA to retract its approval. As new evidence has emerged, some state governments have taken precautionary action despite the federal government’s hesitancy to regulate BPA more stringently. Starting in 2009, Minnesota, California, Connecticut, Hawaii, Illinois, and other states introduced BPA bans (Barraza 2013). These laws primarily targeted baby bottles and sippy cups and, in some cases, child food containers more generally. Within the federal government, there have been conflicting evaluations. In 2009, Senator Charles Schumer and several other politicians in Congress tried to enact the BPA-Free Kids Act of 2009, which would have eliminated BPA from all child food containers, including cups, bowls, and drinking straws (Barraza 2013). By contrast, the FDA repeatedly re-affirmed between 2008 and 2012 that BPA was safe. Understanding why the regulatory and policy debate over BPA is so polarized requires looking into how the chemical regulatory process works to evaluate toxicity risks. We need to briefly review how risk assessment has traditionally worked, and how this analysis struggles to accommodate health effects that depart from a standard model of chemical risk. We use the United States as our example, but most industrial countries and some emerging economies have had similar regulatory systems in place. 3.1 Characterizing BPA risks in the United StatesIn the US, regulators working at FDA, EPA, or CSPC typically assess a chemical’s risk in four steps (see Figure 1). Their risk assessments studies are information-intensive and time consuming. They depend greatly on the availability of relevant scientific studies and the protocols that regulatory institutions use to draw conclusions from these studies about what is ‘safe’ or ‘hazardous’.
Figure 1. The Four Step Risk Assessment Process. Hazard identification involves reviewing data from toxicology and epidemiological studies to ascertain what adverse health effects may result from human exposure to the chemical. Toxicology studies use tests of animals in the laboratory to determine whether exposure can cause higher levels of a particular health endpoint – like cancer or infertility – and then extrapolate from these tests what the risk would turn out to be for humans. Such extrapolations, of course, are inherently uncertain because rodents and humans are not perfectly biologically comparable. Epidemiological studies search for disease patterns in human populations, often comparing between two or more groups of people in different geographical locations to uncover environmental contributions to human diseases. Although epidemiological studies can provide suggestive evidence, they cannot provide ‘absolute proof’ that exposure to a chemical causes a health endpoint. Especially in the US, researchers have focused on whether BPA causes mortality from cancer. This emphasis is built into the entire risk assessment framework, because legislators, regulators, and scientists have prioritized cancer over all other potential health effects during the past 50 years.[1] Because of this historical legacy, the methodologies and assumptions that have accreted around testing for cancer are not designed to readily detect other types of health effects, or to accept that dose-effect relationships may vary enormously between different types of disease mechanisms. Even cancer – once viewed as a single disease – is now understood to be a fiendishly complex set of diseases with diverse physiological and genetic pathways. The traditional testing methods used on animals in toxicology studies measure carcinogenic effects over multiple, sequential stages. Beginning with a biological assay, scientists determine whether a chemical in question causes mutagenic effects on bacteria. If mutations are observed, additional studies are carried out on laboratory rats or mice to identify the ‘maximum tolerated dose’ (MTD), which is the lowest lethal dose that kills the lab animals. A new group of test animals ingest (or are injected with) the chemical at a dose slightly less than the MTD. After two years (or once the animals have died), scientists count the number of tumors that accumulated in the animals’ organs and compare the results to a control group.[2] In recent years, new science has shown that certain chemicals can disrupt the endocrine system – the cells, glands, and tissues that secrete hormones into the bloodstream. Hormones perform numerous essential functions in the human body, and interfering with these can lead to a variety of health effects including breast and prostate cancer, cardiovascular disease, early puberty, obesity, diabetes, erectile dysfunction, and learning and attention-related disorders (Evanthia et al. 2009). More generally, humans can suffer from lower fertility rates. Such health effects are not part of the traditional toxicology testing regime, in part because it is much harder to screen for them in the laboratory. Within the laboratory, it can be challenging to observe whether a chemical has disrupted an individual’s ‘normal’ hormonal patterns (Olea et al. 2002, p. 49). Studies are required to observe how actual human bodies react to chemicals in the environment. Even so, BPA has been a known endocrine disruptor since 1938 (Vandenberg et al. 2007). Dose-response assessment is the second step in traditional toxicity screening. The goal is to connect different exposure levels (dose) with the likelihood that adverse health effects will occur (response). Regulators have generally used experimental animal testing results to generate a ‘monotonic’ dose-response curve that demonstrates one of two key indicators. Either the ‘No Observed Adverse Effect Level’ (NOAEL) or the ‘Lowest Observed Adverse Effect Level’ (LOAEL) is calculated. This calculation allows regulators such as FDA to extrapolate the results to human populations and thus classify a chemical as carcinogenic or not. A dose is considered safe for humans if it falls below the point of NOAEL or LOAEL. Doses that fall higher on the monotonic curve are more toxic. Based on this level, regulators can calculate the reference dose: an estimate of the daily oral exposure level for the human population that is unlikely to result in an adverse health effect over a lifetime.In the 1980s, EPA and FDA decided that BPA was non-carcinogenic, using this dose-response framework. They based this decision on an early study in 1977 that found no convincing evidence of carcinogenicity. The study was conducted by a private laboratory, contracted by the National Cancer Institute to study BPA for carcinogenic effects (Vogel 2009). After two years of administering high doses of BPA to male adult rodents, researchers found BPA’s general toxicity to be low because BPA metabolized rapidly in the animals. Regulators and scientists therefore assumed that BPA must be safe and did not inquire further into its safety for many years. Yet, the National Cancer Institute and other early toxicology studies of BPA did not test estrogenic compounds. They failed to study female animals, and they did not think about the possibility that humans might be exposed continually to these chemicals. Quick metabolism may not mean ‘no risk’ if it is occurring repeatedly. This lack of scrutiny began to change as endocrinology science matured. Endocrinologists studying in utero exposure to synthetic estrogens exposed pregnant mice to low doses of BPA – much lower than in animal studies used in the 1977 NCI regulatory study – and surprisingly observed adverse health effects (Vogel 2009). For instance, a 1990 study showed that male mice embryo exposed to low doses of BPA are more likely to have an enlarged prostates as adults, compared to mice not exposed to BPA (vom Saal et al. 1990). Studying the effects of chemicals crossing the placenta during pregnancy created a new toxicological paradigm that challenged the traditional assumption of ‘the higher the dose, the greater the harm’. The dose-response relationship for endocrine disruptors actually follows a U-shape curve, in which low doses and extremely high doses produce the greatest harm (Welshons et al. 2003). Scientists call this curve a ‘non-monotonic response’. Thus, for endocrine disruptors like BPA, the traditional dose-response assessment fails to accurately assess risk. Exposure assessment, the third step, involves estimating the actual levels at which humans are exposed to a chemical. For BPA, exposure assessment has tended to focus on food consumption patterns, the occurrence of BPA in foods, and non-dietary sources of BPA exposure. Given the range of consumer products made with polycarbonates and epoxy resins, humans are typically exposed to many sources. Food is considered the greatest source of BPA exposure for most population groups because of food packaging.Studies indicate, for instance, that BPA can migrate from polycarbonate packaging into food and beverages. This is because polymerization reactions always leave some monomer unreacted. The unreacted portion of BPA stays solid at room temperature, but over time and under higher temperatures, it can leach out (UK Food Standards Agency 2001). Most government exposure assessments have found that BPA migration from polycarbonate into food is very low under typical room temperature conditions, around 5 micrograms per kilogram (µg/kg) or less of body weight per day (Biles et al. 1997, Mountfort et al. 1997). However, certain populations experience greater exposure, namely infants at 0-6 months age who are fed from polycarbonate bottles that are often heated above room temperature to warm liquid baby formula. For bottle-fed infants, BPA exposures are much higher than for infants fed using non-polycarbonate bottles. BPA can also potentially migrate from epoxy resin can coatings into food and beverages. Nonetheless, food packaging is not the only major source of BPA: thermal paper, plastics, and electronics could also be other sources, and they are only beginning to be included in exposure studies. People can be exposed to many synergistically interacting sources, thus creating a cumulative low dose that can cross a critical threshold. Regulators, industry, scientists, and environmental NGOs disagree on whether and how to include this dimension. Finally, risk characterization determines what exposure level is ‘safe’ for the public. In the face of this new research, regulators have struggled to determine what scientific research should be used to define BPA safety. Between 1997 and 2005, at least 115 studies were conducted by public and private research laboratories in the US, Japan, and Europe, which report a range of adverse effects from various BPA exposure levels (Vogel 2009, p. S562). Many government-funded studies conclude BPA could affect human development, even in small amounts. Industry-funded studies cast doubt on these findings, questioning whether studies that test for estrogenic activity are methodologically sound (raising complaints of lack of reproducibility, poor design, potential confounders unaccounted for, inappropriate manipulation of data, flawed statistical analysis, and so on). These claims hinge on the assumption that traditional toxicology science is the only legitimate way for assessing risk. To provide apparent clarity on the state of BPA science, the Harvard Center for Risk Assessment published, in 2006, a review study of all published studies on BPA, and concluded that only two studies – both of which were funded by industry – provided ‘reliable’ data, in large part because many studies diverged from the traditional monotonic dose-response assessment paradigm. However, the Harvard Center has a history of favoring industry perspectives on risk. By contrast, in 2007, the National Institute of Environmental Health Sciences released findings from two government-sponsored review studies of the same scientific literature. A special expert panel told the institute that BPA concentrations in the human body are associated with "changes in the prostate, breasts, testis, mammary glands, body size, brain structure and chemistry, and behavior of laboratory animals" (vom Saal et al. 2007, p. 134). Thus, the conflicting interpretations of the science continued to play out in regulatory circles. 4. Who is responsible for acting on BPA Risk?In the US context, there have been ongoing debates about who should take responsibility for reducing any BPA risks. Should it be government with its regulatory authority and public protection role? Or should it be industry with its practical capacity to change products and its interest in profit? Should intervention be left to consumers to decide through the market whether they want to bear what might be a speculative risk? In the 1970s, the answer would have been clear: the federal government had a political stake in defending its population from environmental degradation, and was expanding its regulatory apparatus to enable strong precautionary action. But by the 1980s, when the Reagan Administration’s deregulatory agenda was in full flow, the answer would have been: let industry and the market choose. In the 2000s, regulation was more welcome politically, but some government agencies still favored industry interests after decades of lobbying and Congress pressures. FDA’s story illustrates the entrenched institutional cultures and constraints that shape US chemical regulation. Historically, FDA was meant to protect Americans from adulterated and contaminated foods (as well as assuring pharmaceutical safety). But FDA became caught between its dependence on traditional risk assessment methods and its closeness to industry. FDA retained considerable discretion to interpret scientific research to support its regulatory cases. Nonetheless, in the 2000s and 2010s, fissures have opened up inside FDA, resulting in confused positions that reveal much about its organizational thought processes. Given the inertia of a regulatory system founded on traditional toxicology principles, it was little surprise that in 2008, FDA agreed with the Harvard Center’s conclusion that BPA was safe at current exposure levels. While some retailers voluntarily removed products containing BPA from their shelves, FDA cited the lack of validity of low-dose studies that rely on questionable scientific methodologies that may not be reliable enough for regulatory toxicity testing. This finding undercuts attempts by federal legislators to intervene more vigorously. The Consumer Product Safety Modernization Act had just passed in Congress. Inspired by state-level and European Union bans on phthalates, this law empowered the Consumer Product Safety Commission to ban, for use in children’s soft toys, a handful of phthalates that were suspected of being significant endocrine disrupters. This law would have included a similar ban on BPA in children’s food containers. From 2009 onwards, FDA began to revise its position on BPA. It is not a coincidence that the Obama Administration entered power in 2009. Like other government agencies, political appointees oversee FDA, altering with each Administration. Thus, politically contentious regulatory decisions may change according to the values of each Administration. The agency requested that an independent science advisory board review BPA findings (yet again) and make recommendations. By 2010, FDA officially began expressing concerns about BPA safety. In 2012, FDA rebuffed a petition from the Natural Resources Defense Council to ban BPA in food packaging and containers as no longer ‘generally accepted as safe’. Oddly enough, the agency announced that baby bottles and children’s drinking cups should no longer contain BPA. FDA explained that this voluntary call was not based on scientific evidence but on a request by the American Chemistry Council (the chemical industry’s main trade association) to implement the ban in order to boost consumer confidence in the midst of regulatory confusion (Tavernise 2012). The controversies over BPA research continue on. A 2012 review of more than 800 studies on BPA found that even extremely small doses of BPA can be toxic and that low doses of BPA are linked to higher rates of obesity, diabetes, thyroid disease, breast cancer, prostate cancer, and other illnesses (Vandenberg et al. 2012). The study authors conclude that "fundamental changes in chemical testing and safety determination are needed to protect human health" (ibid.). Yet, in February 2014, FDA scientists concluded that low-level exposure to BPA is safe, and that low-dose studies did not show adverse effects (Delclos et al. 2014). However, some scientists not affiliated with the FDA study criticize the FDA’s study design for not investigating all relevant health endpoints like changes in brain behavior (Bienkowski 2014). Researchers from another government study organized by the National Institutes of Health charge that FDA did not use the most up-to-date quality control methods in use among university researchers, nor did it incorporate new scientific findings about how chemicals affect human bodies through endocrine disruption (Blake 2014). Academic researchers and advocacy groups criticize the FDA of being too accommodating to the scientific standards of industry scientists, whose real aim is to institute a standard of proof for risk that is unattainable – a tactic that the tobacco industry used to fight regulation. Proponents of BPA’s safety respond that academic scientists relying on large government grants have a vested interest in keeping BPA on the publicly-funded research agenda, and that their research is overly influenced by personal goals of doing ‘advocacy research’ that supports activists’ claims (Miller 2014). In these highly adversarial circumstances, scientific evidence will remain contested, and it seems unlikely that a decisive ban on BPA is forthcoming unless government strongly favors a precautionary stance. In the absence of federal regulation, and with a patchwork of a few states restricting BPA through bans or labeling laws, what can be done? Some companies have attempted to address consumers’ concerns about BPA through product innovation. 5. Substituting BPA for Alternatives in Well-Established Production ChainsIn response to consumer concerns, NGO campaigns, and imminent regulation in the late 2000s, manufacturers quickly introduced new product lines, marketing them as containing BPA-free materials. As scientific evidence pointed to BPA’s endocrine-disrupting effects, consumer safety, environmental health, and disease-advocacy NGOs built campaigns targeted against retailers and brand name products. These campaigns provided information to consumers about BPA risks and urged them to boycott products known to feature BPA. For example, the Breast Cancer Fund advised against buying plastic-coated toys and cooking utensils altogether, with ‘BPA-free’ plastics permissible if necessary. Some consumers began using stainless steel bottles and glass bottles as alternatives to plastic bottles. The SIGG scandal in 2009 further intensified the debate over whether BPA should even be allowed to be present in bottles (Baker 2009). SIGG, a stainless steel bottle manufacturer, had claimed that its products were BPA-free, only for independent laboratory testing to discover that the bottle lining contained trace amounts of BPA. A widespread consumer backlash against SIGG was the result. In 2008, the Canadian government imposed a ban on certain uses of BPA in consumer products. Even before the law came into effect, many consumer product manufacturers and retailers removed BPA products from their offerings in Canada. Because of a common industry chain supplying both countries, Wal-Mart Canada, CVS, Toys’R’Us, Playtex, Nalgene, Whole Foods, and other companies in the US and Canada voluntarily stopped selling baby bottles and water bottles made with BPA. Nalgene Outdoors Products, based in Rochester, exemplifies this response to market pressures and regulatory risks. Steven Silverman, the firm’s manager, said: "Based on all available scientific evidence, we continue to believe that Nalgene products containing BPA are safe for their intended use. However, our customers indicated they preferred BPA-free alternatives, and we acted in response to those concerns" (Austen 2008, p. C1). To stay in the bottle business, Nalgene introduced a line of bottles made from Eastman Chemical Company’s Tritan copolyester. As this action suggests, product manufacturers began investigating BPA substitutes in response to retailers pulling BPA products from their shelves. A number of companies have turned to existing functional equivalents of BPA to develop their own BPA substitutes. Bisphenol S (BPS) is a popular BPA substitute, which boasts similar product attributes as BPA but with less risk of leaching from with heat or sunlight.[3] BPS was first made in 1869 as a dye, but did not find commercial application until 2006 as a substitute for BPA in paper products such as cash-register receipts, airplane luggage tags, and boarding passes. BPS can now be found in products made from recycled paper, like pizza boxes and food buckets. BPA-substitutes also come from outside of the bisphenol family. In 2002, the Eastman Chemical Company began working on a new heat-resistant plastic called Tritan, which it released in 2007. Many product manufacturers quickly began using Tritan instead of BPA-containing polycarbonate to make infant products, water bottles, and food containers. To buttress its claims to safety, Eastman released third-party test results showing that Tritan monomers do not bind to oestrogen or androgen receptors, and is therefore free of estrogenic activity (Eastman Chemical 2010). Yet some recent scientific research indicates that Tritan, BPS, and other BPA substitutes do induce estrogenic activity and therefore pose endocrine disruption risks similar to BPA. In 2011, researchers at CertiChem, a chemical screening firm, reported that 92% of 102 commercially available plastic products (e.g. ‘BPA-free’ plastic cups marketed for children and purchased from Target, Walmart, and Babies R Us) leached chemicals with estrogenic activity (Yang et al. 2011). Scientists at the University of Texas Medical Branch in Galveston found that BPS, like BPA, disrupts the endocrine system at extremely low doses, noting the similar size and structure of both chemicals, which both have the potential to bind to natural oestrogen receptors inside cells (Viñas et al. 2013). These studies are troublesome in light of biomonitoring studies that show that even though BPS has only been in use for less than a decade, it is already pervasive in human populations: 81% of 315 urine samples from men and women in the United States and seven Asian countries contained BPS (Liao et al. 2012). In 2014, EPA’s ‘Design for the Environment’ program, which seeks to identify safer alternative chemicals, released a report assessing 19 chemical alternatives to BPA used in receipt paper, including BPS (US EPA 2014). The report found that BPS poses similar risks to public health as BPA, concluding that all of the BPA alternatives are associated with some trade-offs. Because the major US chemicals policy, the Toxic Substances Control Act (TSCA), has allowed new chemicals to enter the market without being tested for safety, BPA substitutes have been offered to consumers with no or little public research available on the real or potential risks of such substances. 6. Analyzing the Ethical Situations of Moral Actors Regarding BPAAs BPA has emerged as a chemical of concern over the past 15 years, many actors in the chemical industry, government, and civil society in the US have wrestled with the difficult dilemmas that using possibly harmful chemicals in products can pose. In deciding what to do, these actors have most often taken three major ethical stances. First, actors can employ deontological reasoning. Deontological theories determine moral action according to intention, or whether an action is done for the right reasons. The word ‘deontology’ derives from the Greek words for duty (deon) and science (logos). Deontologists assert that people have a duty to do the right thing no matter the consequences. Yet, deontologists differ in how they define what constitutes ‘good’ or ‘right’ action. For example, the German philosopher Immanuel Kant (1724-1804) asserted that because what is virtuous is not always identifiable, we must instead do ‘right’ (Hinton 2002). For Kant, to determine what ‘right action’ is, one must consider whether the action would be desirable as a ‘universal law’. For example, the precautionary principle is sometimes invoked as a deontological base for environmental laws (Kysar 2010). According to deontologists, societies should prioritize human and environmental welfare ahead of economic profit as a universal norm of justice. Protecting human well-being is a fundamental societal value. If there is a possibility of serious, irreparable injury to human health occurring, even if scientific proof remains uncertain, then societies should intervene to prevent that harm. Second, actors can rely on consequentialist ethics to guide their choices. This ethics holds that the consequences of an action will determine whether it is morally permissible or not. Moral action, then, is that which produces ‘right consequences’. Utilitarianism, the most influential form of consequentialism, applies the principle of utility to determine ‘right consequences’. The morally right action is the one that produces the most utility (‘the greatest good for the greatest number’). A strict utilitarian gives equal weight to each person’s well-being in assessing utility, ignoring whether an action has a negative impact on specific subgroups of society. However, John Stuart Mill (1806-1873) proposed balancing utilitarianism with a set of fundamental rights that defend individual liberties, namely the right to protection from harm, free speech, free association, and self-determination (Mill 1859). Mill’s merger of utilitarian moral theory with liberal political philosophy suggests that a moral society is comprised of individuals who are free to pursue their personal goals and interests, unless such pursuits harm another person. Over the last century, utilitarian calculus has come to dominate decision-making in government and business organizations. The practice of cost-benefit analysis (CBA) operationalizes Mill’s liberal utilitarianism by measuring all of the costs and benefits of a policy in economic terms, applying weights to different costs and benefits, and comparing the totals, with the overall objective being to maximize the ratio of total costs to total benefits. This utilitarian calculus requires that the calculator adopt an impartial ‘view from nowhere’ in assessing all the costs and benefits for a range of possible actions. Thus a chemical industry consultant suggests: "The really prudent step is to make the best scientific evaluations of the risk from the product as compared to the risks and loss of benefits associated with removing it from the market before any actions are taken" (Entine 2011, pp. 19-20). Finally, actors can choose to ostensibly sidestep ethical questions and instead engage in technical arguments over how to interpret the scientific evidence. Companies, regulators, and legislators frequently portray science as an arbiter of objective, factual truth. By doing so, they remove social and political values from debates over chemical risks, and emphasize issues of methodological rigor, data quality, and expert credibility as the fundamental ones (Kinchy 2012). If other actors attempt to raise concerns about the underlying priorities of chemical manufacturing, or about the observation that chemical exposures are widespread, they are attacked as lacking in scientific rigor and biased. Actors who take a ‘scientized’ perspective fail to recognize that they, too, are often cloaking social and political values under a veneer of objectivity (ibid.). We will now consider several key actor groups and summarize their potential reasoning. Our approach is to consider the practices and statements of these actors as they respond to the BPA situation. 6.1 Business managers and corporationsBusiness executives must ponder whether their company should eliminate BPA or continue using it. At this time, they have access to incomplete, uncertain information about BPA risks, so they must necessarily be speculative. Because of their organizational culture, managers are likely to use utilitarian reasoning to evaluate the costs and benefits to their company in taking a particular course of action. This analysis can vary between managers as well as different types of companies. A firm manufacturing BPA stands to lose billions of dollars worth of sales over a few decades, since the substance has one of the highest production volumes worldwide. The firm could switch to producing substitutes for BPA to replace this lost market. But finding suitable alternatives and re-engineering product design will require millions of dollars in R&D. Moreover, once alternative ingredients are found, new testing must be done to ensure those substances are safe, and new supply chain partners must be cultivated and vetted. Whereas BPA is already grandfathered under the Toxic Substances Control Act, new chemicals may face more searching scrutiny from EPA. The company could undertake all of these activities only to lose its market-share to competitors who are not taking similar action. To oppose any change, managers could point to the existence of weak scientific evidence that shows low-level exposure to their product causes future adverse health effects. From their perspective, hypothetical harm is not enough to warrant real financial risk. Exiting the BPA market could violate a company’s fiduciary duty to its shareholders, to whom they are obligated to make sound financial decisions. From this view, it would be morally permissible for a company to wait for clearer scientific evidence, new regulations, or sizeable consumer demand for safer alternatives. In contrast, a retailer or a consumer product manufacturer might face a more complicated calculus. On one hand, ‘downstream’ firms can decide to trust that the chemical industry has public health interests at heart. There is no need to worry about possible BPA risks, since the chemical has already been on the market for decades, and any latent health consequences would have appeared by now if they were going to. Moreover, BPA has been approved by US government regulators for use in products. Many firms appear to require stronger government positions on BPA before they are willing to intervene. Such a stance effectively sidesteps the company’s ethical responsibility, if any, by assigning it to scientists and governments. For example, the Coca Cola Company’s position on BPA maintains the status quo in the absence of regulatory action: While we are very aware of the highly publicized concerns and viewpoints that have been expressed about BPA, our point of view is that the scientific consensus on this issue is most accurately reflected in the opinions expressed by those regulatory agencies whose missions and responsibilities are to protect the public’s health. The consensus repeatedly stated among regulatory agencies in Australia, Canada, Europe, Germany, Japan, New Zealand and the United States is that current levels of exposure to BPA through food and beverage packaging do not pose a health risk to the general population. [Coca-Cola 2012, p. 38] The downstream company could also decide to apply utilitarian reasoning in the same way that a BPA manufacturer might. There would be costs from changing a product to be BPA-free, such as sourcing new raw materials, reformulating designs, and adjusting manufacturing equipment to use BPA substitutes. Manufacturers might encounter retailers which are reluctant to change their product lines. In evaluating the merits of eliminating BPA, firms could skeptically ask: how many human lives will be affected by health problems? Are adults dying from cancer, or are children suffering from development problems such as undescended testes? Or are children afflicted by ‘only’ small cognitive impairments like memory fragility? Companies may argue that too few people will be seriously injured to warrant the considerable costs of transition to safer alternatives. They may treat their ethical conundrum as a business decision. Yet downstream firms might benefit from maintaining or even expanding their markets as consumers demand safer products. They could acquire a reputation for protecting consumer well-being that translates into higher share prices, better employee morale, and government goodwill in setting standards. The firms may avoid legal and regulatory liability for continuing to use BPA. Even if courts are not yet ruling in lawsuits that BPA products harm consumers, there is arguably enough scientific evidence of BPA risks now to find that companies should have known about these risks and were negligent in failing to act to prevent them. Nonetheless, business managers and companies do not have to adopt utilitarian reasoning. They may use deontological reasoning to declare that the risks of BPA call for precautionary action as soon as a threshold of sufficient scientific evidence of potential harm is passed. That is, they do not have to await a decisive ruling or regulatory order before acting voluntarily to invest in innovation or to phase out BPA. Still, there can be wide differences of opinion as to what the appropriate threshold of scientific evidence should be. It could vary from ‘probable risk’ to ‘reasonably likely risk’ and to ‘plausible but not likely risk’. 6.2 Chemists and designersMost chemists and designers who work with BPA and other endocrine disrupting chemicals in consumer products do so within an industry or corporate context. Therefore they are subject to similar pressures and considerations that managers have to face. They may adopt utilitarian reasoning along the same lines as discussed just above. They may decide that they ought to help maintain their employer’s business, ahead of any ethical issue. Indeed, they may think that they could lose their jobs if their employer does not thrive, or frowns on their ‘whistleblowing’ as regards chemical risks. They can put their personal welfare ahead of public welfare. Nonetheless, chemists might believe that they have a duty to protect vulnerable populations such as children and pregnant women when designing molecules. Like business managers, they may have their own families and children to worry about, and they do have the moral agency to make fundamental design choices. Here, chemists can decide whether they have a deontological obligation to implement green chemistry principles, which include designing out toxicity from chemicals where practicable, designing safer products, maximizing atom efficiency, using renewable feedstock, designing chemicals to degrade readily (Anastas & Warner 1998). However, the vast majority of chemists are not trained in environmental health, green chemistry, or even toxicology, so they can struggle to carry out green chemistry principles. They may not even know that the green chemistry field exists. Thus their moral agency is inhibited by their professional and organizational conditions. In practice, a number of product designers likely have raised significant concerns about BPA from within chemical and product manufacturers. They can point to emerging scientific evidence of estrogenic activity as a reason to intervene. However their ability to do so may rests on the availability of chemical substitutes that are reliably safer. In this regard, product managers can support the development of improved information tools like the EPA’s Safer Chemical’s list and independent or non-profit organizations that offer testing services to identify which materials leach chemicals with estrogenic activity. 6.3 Regulators and legislatorsIn the US, chemicals regulation is based on a utilitarian framework that requires government officials, companies, and other actors to focus on the question of whether material convenience outweighs physical harm. The Toxic Substances Control Act has hamstrung officials at EPA when it comes to trying to regulate harmful chemicals. On one hand, regulators were obliged to prove that a chemical posed substantial risk to human health, the benefits of a regulatory action outweighed the burden to industry and society, and the action was the most reasonable possible. In practice, regulators had to compile massive amounts of evidence, using cost benefit analysis and risk assessments, to justify any ban. This situation resulted, in part, from an US Court of Appeal ruling in 1989 that EPA did not provide enough evidence to warrant an asbestos ban. On the other hand, regulators lacked power to require companies to carry out even basic toxicity screening of chemicals, so they were usually unable to prove that a risk existed. Thus otherwise sympathetic officials were unable to escape their regulatory confines. By contrast, since the 1990s, EPA officials have become more willing to enforce the new substances review program, in which new chemicals are evaluated more closely for their health effects. But the number of existing chemicals far exceeds new chemicals: some 62,000 were already permitted under TSCA in 1976. In recent years, EPA’s leadership has ordered the agency to tackle chemicals more aggressively, a call grounded in the precautionary principle. For example, in a 2009 speech at the Commonwealth Club in San Francisco, the head of the EPA, Lisa Jackson, declared: "We need to review all chemicals against safety standards that are based solely on considerations of risk – not economics or other factors – and we must set these standards at levels that are protective of human health and the environment" (Jackson 2009). Even so, existing chemicals – of which BPA is one – readily evade review. In response, regulators could decide to create voluntary programs to coax industry into doing more toxicity testing of chemicals and possibly phasing out especially harmful ones. Whether or not regulators actually engage in this work is an ethical choice. EPA officials may rely on utilitarian reasoning: is trying to build a BPA program likely to be politically or economically costly, compared to the health benefits? Will there be strong push-back from powerful companies? Or, the officials could invoke deontological reasoning and prioritize the voices of those people who are most affected by exposure to BPA, like children and mothers. In turn, legislators are elected in a political environment in which donations and lobbying from industry interests have been rampant since the 1980s. This is particularly true at the federal government level where the chemical industry has pervasive influence. Members of Congress thus face ethical quandaries: should they reject donations from the chemical industry and jeopardize their chances of re-election? Or should they heed calls from parents who are becoming more worried about chemical risks? Should more ecologically conscious Senators compromise their values and reach an agreement with industry-friendly Senators in order to reform the Toxic Substances Control Act? Or should they hold out for more stringent rules that are less likely to become law? In many cases, legislators (and regulators) simply decide to follow the status quo. They are unwilling to abandon their dependence on campaign finance. They can follow the lead of many companies in ignoring ethical questions altogether and emphasizing the need for more science. This behavior is seen in the 2008 Congressional hearing on BPA, in which most industry and government witnesses avoided debating the ethical issues at stake. Instead, they sparred over whether or not scientific evidence existed to justify regulatory action. As Marian Stanley of the American Chemistry Council testified during the 2008 Congressional hearing: "In the past 2 years comprehensive scientific assessments from the European Union, the U.S. National Toxicology Program, Health Canada, NSF International, and the European Food Safety Authority have all been undertaken, and these assessments support the continued safe use of consumer products containing BPA" (US Congress 2010, p. 81). In other words, few participants at the hearing (apart from Dr. Ted Schettler, a NGO scientist) brought up ethical questions such as: why are humans being exposed to harmful chemicals at all? Who is responsible for putting us into this predicament? Why not make safe products to begin with? What are the views of those people who are harmed the most? They remained trapped within a risk assessment paradigm that favors seemingly objective and neutral analysis. Yet, legislators can decide to take moral leadership by introducing new laws that force industry to remove BPA and other substances from products. In the US, this has tended to happen more often at the local and state government levels, which can be less subject to industry influences. As of 2015, at least 12 states from California to Massachusetts had passed some sort of restriction on BPA use (National Conference of State Legislatures 2015). Most of these laws banned the manufacture and sale of drinking bottles that contain BPA, if they were intended for use by young children. One law, in Minnesota, banned all uses of BPA in food containers for use by children. Moral leadership can also happen at the federal government level. In 2015, lawmakers introduced the BPA in Food Packaging Right to Know Act, which would make it illegal to sell food in containers with BPA unless the container is labeled with the statement: "This food packaging contains BPA, an endocrine-disrupting chemical, according to the National Institutes of Health."[4] This law, however, simply creates information aimed at consumers, who are obliged to decide whether they are willing to accept the risk of buying a potentially harmful product. It arguably shifts moral agency from firms to consumers. 7. ConclusionsBy studying the BPA case, chemistry students can learn several critical lessons for use in their future careers. The full health and ecological impacts of chemicals may only appear long after widespread use in products begins. BPA use in products has become ubiquitous and many millions of people are exposed to BPA. Although BPA’s estrogenic properties have been known since the early 1900s, whether its estrogenicity is harmful remains hotly debated. Faced with scientific controversy, government and company decision-makers may struggle to recognize and act on the emerging risks. Failure to address the potential risks of BPA has implications for the numerous chemicals used in consumer products that are suspected of being endocrine disruptors. Companies are not inherently unethical in having helped disperse harmful chemicals into society if they could not foresee the health consequences. However faced with new knowledge about product risk, companies may act unethically if they persist in making or using these chemicals after evidence emerges signaling cause for concern (European Environmental Agency 2001). With BPA, sufficient warning signals arguably exist now to justify the trade-off between ethical action and financial costs. As we have seen, difficult ethical dilemmas exist when deciding whether sufficient scientific evidence exists to warrant actions such as reformulating products, removing chemicals, or regulating substances. Should precautionary action be taken, even though this might jeopardize a firm’s markets and profits? Or should the status quo be retained even though it might cause harm to some people? Deontological and consequential ethics theories are often applied to resolve these debates. The diverse values that people hold can color their thinking; calculations can vary widely. For example, Nalgene used utilitarian analysis to decide to remove BPA from its bottles, reasoning that the benefits of doing so would outweigh the costs. Yet, other firms did not withdraw BPA, arguing that the economic costs of changing to an alternative substance would be too large. And the American Chemistry Council, joined by many companies, argued that more science was required to prove that a BPA risk existed, or that regulators had certified BPA as safe, implying that ethical questions were irrelevant. Numerous actors involved in chemicals production and use have moral agency because of their ability to influence chemical product design. They can be held ethically accountable for their actions and the consequences of those actions. They can be inside firms, in government departments, and scattered across civil society. Chemists are not the only actors who can make ethical choices that matter, and they can find allies in many places inside and outside industry. These allies can be sympathetic managers, other firms, regulators, and environmental NGOs who want to make chemicals safer. Civil society involvement can also increase scrutiny of the choices of corporations and chemists regarding toxic chemicals. The work of activists such as the Breast Cancer Fund and Clean Production Action can help improve decision-making through critically appraising why companies are using BPA in products. The BPA case is only one example of a growing number of chemical risks calling for an industry response. Other substances of concern include phthalates, flame retardants like polybrominated diphenyl ethers (PBDEs), and perfluorinated compounds such as perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). These chemicals raise similar concerns through their ability to damage human development and reproduction; they can also cause certain kinds of cancers. Phthalates are commonly found in soft toys and cosmetics; flame retardants are everywhere from electronics to furniture; PFOA is used in teflon-coated pots and Goretex clothes. As we saw, in trying to substitute apparently safer chemicals for known or suspected toxins, firms can choose substances that are little better due to lax regulatory screening. As this case makes clear, the way forward is difficult. Removing toxic chemicals from consumer goods is a vast political, economic, and design challenge that poses many complex ethical dilemmas for the diverse stakeholders involved. Better understanding of the ethical dimensions of creating safer consumer goods can help lay the foundation for opening up better communication between regulators and legislators, company managers, chemists and product designers, consumers, and the public. By helping all actors develop deeper analysis of the ethical values at stake and the underlying moral commitments that they share with one another, they can create a safer, more sustainable chemical industry, even with imperfect science. You can play an important part in this process through whatever you end up doing. Notes[1] In 1958, for example, Congress enacted the Federal Food, Drug and Cosmetics Act, which included the now-repealed ‘Delaney Clause’. This provision effectively required FDA to prohibit food additives and pesticide residues that were "found to induce cancer when ingested by man or laboratory animals". The Delaney Clause was repealed in 1996, but not before 40 years of toxiciological testing entrenched carcinogens as a fundamental concern. [2] For example, if the control group has an average of one tumor per animal and the test group has an average of four tumors per animal, the chemical is said to increase human cancer incidence by 300 percent. [3] A BPA molecule consists of two phenol groups connected by a branched three-carbon group, whereas a BPS molecule has two phenol groups connected by a sulphone group (-SO2). [4] The bill has been introduced to committee but no hearings have been conducted. See: https://www.congress.gov/bill/114th-congress/senate-bill/821. ReferencesAnastas, P.T. & Warner, J.C.: 1998, Green Chemistry: Theory and Practice, New York: Oxford University Press. Austen, I.: 2008, ‘Bottlemaker to Stop Using Plastics Linked to Health Concerns,’ New York Times, April 18, C1. Baker, N.: 2009, ‘Why I’ll Swig From My Sigg Bottle No More’, Huffington Post, Sept 26, available at: http://www.huffingtonpost.com/nena-baker/why-ill-swig-from-my-sigg_b_269603.html, accessed 21 Oct. 2016. Barraza, L.: 2013, ‘A new approach for regulating bisphenol A for the protection of the public’s health’, The Journal of Law, Medicine & Ethics, 41 (1), 9-12. [not cited in text] Bienkowski, B.: 2014, ‘New BPA Experiment Finds No Low-Dose Effects’, Environmental Health News, February 13. Available at: http://www.environmentalhealthnews.org/ehs/news/2014/feb/bpa-low-doses, accessed 21 Oct. 2016. Biles, J.A.; T.P. McNeal, T.H. Begley & Hollifield, H.C.: 1997, ‘Determination of Bisphenol A in reusable polycarbonate food-contact plastics and migration to food simulating liquids’, Journal of Agricultural and Food Chemistry, 45, 3541-3544. Blake, M.: 2014, ‘Scientists Condemn New FDA Study Saying BPA Is Safe: "It Borders on Scientific Misconduct"’, Mother Jones, March 24, available at: http://www.motherjones.com/environment/2014/03/scientists-slam-fda-study-bpa, accessed 20 April 2016. Calafat A.M.; Ye X., Wong L.Y.; Reidy J.A. & Needham L.L.: 2008, ‘Exposure of the US population to bisphenol A and 4-tertiary-octylphenol’, Environmental Health Perspectives, 116 (1), 39-44. Case, D.: 2009, ‘The Real Story Behind Bisphenol A’, available at: http://www.fastcompany.com/1139298/realstory-behind-bisphenol, accessed 21 Oct. 2016. Centers for Disease Control: 2009, Fourth National Report on Human Exposure to Environmental Chemicals, available at: http://www.cdc.gov/exposurereport/, accessed 21 Oct. 2016. . Coca-Cola Company: 2012, ‘Sustainability Report 2011/2012’, available at: http://www.coca-colacompany.com/sustainabilityreport/me/product-safety-and-quality.html#section-cocacola-and-alcohol-ethanol, accessed 21 Oct. 2016. Delclos, B.K.; Camacho, L.; Lewis, S.M. & Vanlandingham, M.M.: 2014, ‘Toxicity evaluation of bisphenol A administered by gavage to Sprague-Dawley rats from gestation day 6 through postnatal day 90’, Toxicological Science, 139 (1), 174-197. Eastman Chemical: 2010, ‘New Third-Party Test Results Confirm Eastman Tritan Copolyester is Free of Bisphenol A and Estrogenic Activity’, ChemInfo.com, May 13, available at: http://www.chem.info/news/2010/05/new-third-party-test-results-confirm-eastman-tritan-copolyester-free-bisphenol-and, accessed 21 Oct. 2016. Entine, J.: 2011, ‘Scared to Death: How Chemophobia Threatens Public Health’, available at http://www.pavementcouncil.org/pavementcouncil/americancouncil.pdf, accessed 21 Oct. 2016. Evanthia, D-K:; Bourguignon, J-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T. & Gore, A.C.: 2009, ‘Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement’, Endocrine Reviews, 30 (4), 293-342. European Environmental Agency: 2001, Late lessons from early warnings: the precautionary principle 1896-2000, available at: http://www.eea.europa.eu/publications/environmental_issue_report_2001_22, accessed 20 April 2016. Fiege, H.; Voges, H-W.; Hamamoto T. et al.: 2012, ‘Phenol derivatives’, in: K. Othmer (ed.), Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, pp 521-586. Grand View Research: 2014, ‘Global Bisphenol A (BPA) Market Demand Is Expected To Grow At CAGR of 4.7% From 2014 To 2020’, available at: https://globenewswire.com/news-release/2014/11/12/682387/10107790/en/Global-Bisphenol-A-BPA-Market-Demand-Is-Expected-To-Grow-At-CAGR-of-4-7-From-2014-To-2020-New-Report-By-Grand-View-Research-Inc.html, accessed 21 Oct. 2016. Hinton, T.: 2002, ‘Kant and Aquinas on the Priority of the Good’, The Review of Metaphysics, 55, 825-846. Kinchy, A.: 2012, Seeds, Science, and Struggle: The global politics of transgenic crops, Cambridge, MA: MIT Press. Kysar, D.: 2010, Regulating from Nowhere: Environmental law and the search for objectivity, New Haven: Yale University Press. Jackson, L.: 2009, ‘Remarks to the Commonwealth Club of San Francisco’, September 29, available at: https://yosemite.epa.gov/opa/admpress.nsf/8d49f7ad4bbcf4ef852573590040b7f6/fc4e2a8c05343b3285257640007081c5!OpenDocument, accessed 21 Oct. 2016. Jacobsen, H.: 2015. ‘EU’s food safety agency gives green light to Bisphenol A’, available at: http://www.euractiv.com/section/agriculture-food/news/eu-s-food-safety-agency-gives-green-light-to-bisphenol-a/, accessed 21 Oct. 2016. Liao, C.; Liu, F.; Alomirah, H.; Loi, V.D.; Mohd, M.A.; Moon, H.B.; Nakata, H. & Kannan, K.: 2012, ‘Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures’, Environmental Science and Technology, 46 (12), 6860-6866. Mill, J.S.: 1859, On Liberty, London: Parker. Miller, H.: 2014, ‘BPA Is A-OK, Says FDA’, Forbes, March 12, available at: http://www.forbes.com/sites/henrymiller/2014/03/12/fda-research-confirms-bpa-is-a-ok/#6f08bee2714d, accessed 21 Oct. 2016. Mountfort, K.A.; Kelly, J.; Jickells, S.M. & Castle, L.: 1997, ‘Investigations into the potential degradation of polycarbonate baby bottles during sterilization with consequent release of bisphenol A’, Food Additives and Contaminants, 14, 737-40. National Conference of State Legislatures: 2015, ‘NCSL Policy update: State restrictions on Bisphenol A (BPA) in consumer products’, available at: http://www.ncsl.org/research/environment-and-natural-resources/policy-update-on-state-restrictions-on-bisphenol-a.aspx, accessed 21 Oct. 2016. Olea, N.; Fernandez, M.F. & Olea, M.F.: 2002, ‘Human Exposure to Endocrine Disrupters, an Overview’, in: L. Chyczewski, J. Niklinski & E Pluygers (eds.), Endocrine Disrupters and Carcinogenic Risk Assessment, Amsterdam: IOS Press. Ritter, S.K.: 2011, ‘BPA Is Indispensable For Making Plastics’, Chemical and Engineering News, 89 (23), available at: https://pubs.acs.org/cen/coverstory/89/8923cover4.html, accessed 21 Oct. 2016. Tavernise, S.: 2012, ‘F.D.A. Makes It Official: BPA Can’t Be Used in Baby Bottles and Cups’, New York Times, July 17, A15. Turker, M.S.: 2012, ‘Banning Bisphenol A in the United States and Canada: Epigenetic Science, the Precautionary Principle, and a Missed Opportunity to Protect the Fetus’, Journal of Health & Biomedical Law, 8, 173. UK Food Standards Agency: 2001, ‘Survey of Bisphenols in Canned Foods’, March 2001. Available at: http://www.food.gov.uk/science/surveillance/fsis-2001/bisphenols, accessed 21 Oct. 2016. US EPA: 2014, ‘Bisphenol A Alternatives in Thermal Paper’, available at: https://www.epa.gov/sites/production/files/2014-05/documents/bpa_final.pdf, accessed 21 Oct. 2016. US Congress, 2008: ‘Safety Of Phthalates And Bisphenol-A In Everyday Consumer Products’, Hearing Before The Subcommittee On Commerce, Trade, And Consumer Protection, June 10, available at: http://www.gpo.gov/fdsys/pkg/CHRG-110hhrg56091/html/CHRG-110hhrg56091.htm, accessed 21 Oct. 2016. Vandenberg L.N.; Hauser, R.; Marcus, M.; Olea, N. & Welshons, W.V.: 2007, ‘Human exposure to bisphenol A (BPA)’, Reproductive Toxicology, 24 (2), 139-77. Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs Jr., D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; Zoeller, R.T. & Myers, J.P.: 2012, ‘Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses’, Endocrine Reviews, 33 (3), 378-455. Viñas, R. & Watson, C.S.: 2013, ‘Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell function’, Environmental Health Perspectives, 121 (3), 352-358. Vogel, S.A.: 2009, ‘The Politics of Plastics: The Making and Unmaking of Bisphenol A "Safety"’, American Journal of Public Health, 99 (S3), S559-S566. Vogel, S.A.: 2013, Is it Safe?: BPA and the Struggle to Define the Safety of Chemicals, Berkeley: University of California Press. vom Saal, F.; Quadagno, D.; Even, M.; Keisler, L.; Keisler, D. & Khan, S.: 1990, ‘Paradoxical Effects of Maternal Stress on Fetal Steroids and Postnatal Reproductive Traits in Female Mice from Different Intrauterine Positions’, Biology of Reproduction, 43, 751-761. vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.M. & Birnbaum, L.S.: 2007: ‘Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure’, Reproductive Toxicology, 24 (2), 131-138. Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M. & vom Saal, F.S.: 2003, ‘Large effects from small exposures. I. Mechanisms for endocrine disrupting chemicals with estrogenic activity’, Environmental Health Perspectives, 111, 994-1006. Yang, C.Z.; Yaniger, S.I.; Jordan, V.C.; Klein, D.J. & Bittner, G.D.: 2011, ‘Most Plastic Products Release Estrogenic Chemicals: A Potential Health Problem That Can Be Solved’, Environmental Health Perspectives, 119 (7), 989-996. Abigail Martin: |