http://www.hyle.org
Copyright © 2014 by HYLE and Pierre Laszlo
Chemistry, Knowledge Through Actions?Pierre Laszlo*
1. IntroductionThe twentieth anniversary of HYLE: already! I cannot help but be reminded of Alexandre Dumas’s (and Auguste Maquet’s) sequel to The Three Musketeers, ‘Vingt ans après’ (Twenty Years Later).[1] The four heroes are retired, with personas markedly different from those of their youth. The passage of time has matured them. The feeling is melancholic: is there an inevitability to decay? Decay, ageing of a material or a body, are chemical processes. But what is chemistry, for starters? An operational definition then, first: chemistry is what chemists do. Emphasis is on the verb ‘do’, rather than many other possibilities, such as to think, imagine, ideate, ponder, reflect, meditate, work out, cogitate, puzzle over, mull, speculate, ruminate, debate, etc. At the hands of chemists, this science of change, of the transformation of matter is a voluntaristic activity, or set of activities. Not that it eludes rational analysis. In Bachelard’s felicitous phrase, it is a rational materialism. I will not expatiate on the circularity of that definition, chemistry being what chemists do, and chemists being defined in turn by their involvement with chemistry. Indeed, the conceptual bases of chemistry are replete with pairs of concepts mirroring one another (Laszlo 1999). Not only conceptual, practical too: chemistry is both a science and an industry. Due to the latter, billions of people today live in what can only be termed a chemosphere (Russell 1951).[2] The ambivalence of chemistry as both a science and an industry, i.e., part of the advancement of knowledge, but also a money-making and technologies-nurturing business, displays significant porosity, in both directions. Communication of results, or their obfuscation, uses the two channels of publications and patents. Does such an ambivalence between the scholarly pursuit, on one hand, the commercial enterprise on the other, have anything to do with the just mentioned circularity of concepts? Before attempting to answer that question, one may wish to list other ambivalences in what chemists do. 2. The split world of chemistsHere is a short list: they think, not only with their heads, with their hands too. Their technical language, besides a systematic nomenclature, is iconic: chemists rely a great deal, not only on words, on images and diagrams too.[3] Another fundamental dualism is that of structure and dynamics: atomic arrangements of any kind, whether molecules, complexes, supramolecular assemblies, and so on, assume shapes, whose configurations, conformations, etc. chemists make it their job to determine. At the same time, bringing these structures into existence, monitoring their interconversions, goes through running reactions. Work at the bench is inseparable from work at the desk – or in front of a CRT screen. Moreover, the nmr revolution of the 1950s and 1960s made even more blatant the coupling of statics and dynamics, of molecular structure and internal motions within molecules, let alone their interconversions. Microscopic structure and dynamics are the explanans for processes (explananda) taking place in the macroscopic laboratory. The macro/micro dualism is fundamental to chemistry. Chemists think typically about the microscopic entities, while their operations concern the macroscopic states of matter, the reactants, solvents, reagents, flasks, separation devices, etc. In addition, this interplay takes place at a timescale of days, not weeks, months or years.[4] Let me return to the notion of chemists ‘thinking with their hands’. The phrase is decades-old. It probably antedates the book Penser avec les mains (Rougemont 1936). Even though the training of young chemists has changed markedly since I entered the profession,[5] I believe this motto/shorthand description to retain full validity. Thinking with your hands: this seeming oxymoron is reminiscent of these diagrams of yesteryear that dramatically emphasized the huge portion of the brain occupied with the hands. Do MRI studies nowadays bear it out? I have little doubt. But what does ‘thinking with your hands’ mean? Does it refer to the tacit knowledge of chemists, in its extremely diverse manifestations? Since it is tacit, by definition putting it into words is well-nigh impossible. Moreover, one should heed Wittgenstein’s dictum, "about which one cannot speak, one must remain silent" (Wittgenstein 1922, proposition 7). Nevertheless, let us go through a few examples. They have to do, first and perhaps foremost, with risk. Never evaporate a solution to total dryness – lest peroxides in trace amounts explode. Know when to drop what you were doing and duck for protection. Have a fire extinguisher at hand. Translate the start of a smell into an alert. Then, heeding all the messages from one’s hands, such as the rate at which a reaction mixture heats up; feeling the onset of a bubbling, early enough to take steps for preventing it from boiling over; knowing when a reaction is over and the reaction mixture can be safely extracted; perhaps most critically, and this becomes second nature to many a chemist, grasping – a revealing word – a state of equilibrium, in order to turn it to one’s advantage. However, all these instances are far from entailing exclusively ‘thinking with your hands’. At all stages, ‘the head governs work by the hands’, i.e., one takes stock mentally of the perceived changes. Turning now from working at the bench to reading a paper from a fellow chemist, a behavior that connects but does not identify with tacit knowledge, is ‘understanding at a glance’: communication has to be equally transparent as the glassware in the laboratory. Hence recourse to the twin languages of words and images. Chemists are endowed with what can be termed schizovision, i.e., the ability to absorb information both textual and iconic.[6] These two channels, redundant to some extent, show nevertheless separate capabilities. In a typical synthetic publication for instance,[7] the text[8] refers to operations in the laboratory, whereas the images have micro/nanoscopic entities as their referents. What then are the tasks for philosophy of chemistry? 3. On the aims of philosophy of chemistryA trite but true statement: philosophy of chemistry helps to better understand what chemists do, to clarify their goals and to ascertain the meaning of their activity, whether scientific or industrial. A rather outdated aspect is the definition of what to chemists are elements, all several hundred such entities, i.e., a chemical element in a given oxidation state – since oxidation states for a single element differ markedly in their chemistry (a similar statement holds, although more weakly, for isotopes within a given oxidation state). Another, likewise traditional aspect, is to disconnect (as Lavoisier did in the 1780s) chemical nomenclature and terminology from conventional wisdom. Hence, is it really warranted to reiterate that H2O does not fully identify with water in the everyday sense, or, in a more sophisticated manner that goes back to Arrhenius in the 1880s, that the sea does not contain NaCl? (Earley 2005) In a closely related manner, since chemistry is a science, predictably it routinely assails and insults common sense.[9] One of the many tasks of philosophy of chemistry is to identify its founding aporias, in René Thom’s felicitous phrase (Thom 1990). To mention only these, structural chemistry bases itself on the Born-Oppenheimer approximation and upon assuming uniform electronic density on the reticular planes of crystals, for derivation of Bragg Law (Wigner 1949). Dynamic chemistry, in the language of the transition state, bases itself on the metaphor, another aporia, of the Alpine mountain pass between two valleys. Which brings up the critical examination of the relationship of chemistry to neighboring disciplines. Physics, first: it is rather ironical that, even though the interest of physicists[10] in chemistry was short-lived at the turn of the 1930s,[11] this particular relationship continues to account for a majority of the hundreds of papers philosophers of chemistry published during the last 20 years.[12] Currently, chemistry flirts with biology and with material science: these relationships deserve attention. Yet another field of knowledge that chemistry connects with is linguistics, this relationship will reward exploring, if my push in that direction is heeded. Last but not least, chemical practice holds many a worthy question for the philosopher. For instance, what do purification steps entail, ethically, lexically, axiologically, epistemologically, aesthetically? A paper presented related ambitions seven years ago already (Hoffmann 2007). Or, to offer another example, what does ‘running a reaction’ mean exactly? What kind of use of the verb ‘to run’ does it make? The next section will sketch out a linguistic, semiological analysis of routine acts by chemists, it will only hint at its potential productivity. 4. Running a reactionTo run a marathon needs no explanation, it entails running over a set distance without stopping or walking. Obviously, running a reaction differs in kind from such a race. The verb ‘to run’ clearly assumes here a different meaning, metaphorical at least.[13] What do these two meanings share? The notion of a start and a finish, in both processes. Which deserves a little more thought: in an actual race, the runners are let loose by some signal, a pistol shot say; while crossing the finish line, materializing the distance covered, marks the end. With regard to a chemical reaction, the ‘start’ coincides usually with combining the reactants – plus the usual other ingredients such as reagent(s), solvent, catalyst –, stirring to render homogeneous and raising the temperature of the reaction mixture. The ‘finish’ to a chemical reaction entails stopping stirring and heating, removing the mixture from the flask and isolating the products (by operations such as crystallization or distillation). In other words, starting and stopping a reaction are operations in the macroscopic world of the laboratory. Their co-respondents in the microscopic world of molecules are represented, iconically as a rule, by chemical equations, often as mechanistic explanations making use, for instance, of the curved arrow symbolism. In so doing the chemist assumes a Promethean authority over matter or, to resort to another tale from Greek mythology, reaction products are so many daidala (δάιδαλα or δαιδάλεια) (Frontisi-Ducroux 1975). Or, to quote another notion from ancient Greece, chemists resort prominently to metis, this form of cunning personified by Odysseus (Detienne 1974). ‘Running a reaction’, in one meaning, is akin to ‘running a business’, which indeed emphasizes control by the chemist over the contents of the flask. In another, closely related meaning, it resembles ‘running a filly in the Kentucky Derby’, with its dimension of ownership: the chemist owns the species entering the reaction. In yet another meaning of the verb ‘to run’, ‘a river runs its course’: likewise, the chemist monitors the reaction process until its end. Vehicles, such as cars or trains, often ‘run early (or late)’: this other meaning of the same verb emphasizes a mobility, chemical reactions thus are endowed with dynamics and, accordingly, with a mechanism. In yet another meaning, ‘one runs an errand’, i.e., one effects a purchase: ‘to run a reaction’ is also synonymous with ‘to effect’.[14] I respectfully submit that philosophy of chemistry can only be enriched by such a scrutiny of the everyday vocabulary of chemists.[15] Before going on, the allusion to metis induces me to refer to a related description of the art and craft of chemists. They are tinkerers, in the sense that Claude Lévi-Strauss made famous (Lévi-Strauss 1962). 5. Chemists as tinkerersA tinkerer – bricoleur, in French – is someone who makes do with the means at hand (Kauffman 1990). As Claude Lévi-Strauss (1962) put it, "in our own time the ‘bricoleur’ is still someone who works with his hands and uses devious means compared to those of a craftsman." A chemical laboratory is sometimes or often reminiscent of a shop in a flea market, packed with various odds and ends. Lévi-Strauss also wrote: [H]is universe of instruments is closed and the rules of his game are always to make do with ‘whatever is at hand’, that is to say with a set of tools and materials which is always finite and is also heterogeneous because what it contains bears no relation to the current project, or indeed to any particular project, but is the contingent result of all the occasions there have been to renew or enrich the stock or to maintain it with the remains of previous constructions or destructions. [Ibid.] To the tinkerer, re-use is a key mode of operation. An astute recent example comes from the laboratory headed by George M. Whitesides, in the department of chemistry at Harvard. To his research group, bubble wrap is God-given, viz. the interiors of bubbles are sterile: samples can be stored there without need for expensive sterilization equipment. The bubbles of bubble wrap are easily filled with samples by injection with a syringe or a pipette tip, sealing the hole with nail hardener. Transparent in the visible range of the spectrum, the bubbles can be used as ‘cuvettes’ for absorbance and fluorescence measurements. Permeable to gases, bubble wrap can be used to culture and store micro-organisms. By incorporating carbon electrodes, bubble wrap can also be used as electrochemical cells (Bwambok 2014). Examples of such re-use abound.[16][17][18][19] Arguably, the best instance of re-use by chemists is the frequent case of a target molecule from a preparation becoming in turn a tool in another study. The peeling-off procedure for graphene preparation was a chance discovery. It was serendipitous, as scientists like to call such events. Examples of serendipity abound. To mention just one with key instrumental value to chemists, paper chromatography was thus devised, in the context of civil restrictions during World War II. How then should one view tinkering in a philosophical frame, granted that it partakes of metis? Such wiliness or cunning shuttles between a theoretical bent of mind and the utmost practicality: the chemist builds an artificial world in which the Hegelian Spirit can roam (Hegel 1970, Houlgate 1995, Kullmann 1960). 6. Molecular polysemyAre chemists more likely to tinker than other scientists? If indeed so, what explains it? Two distinct but complementary answers offer themselves. The first is that chemical science doubles up as an industry. It has built a chemosphere for humanity. Hence, chemistry, whether as science or as industry, engages in a constant interplay with the everyday. Artifacts made of polyethylene, aluminum foil, or rare earths go back and forth between the home and their re-use in the laboratory. The other answer, and it needs explication, is that chemists, in addition to making new species, also innovate by adding yet more uses to already existing molecules. I will ‘follow’ Wittgenstein here, on adding new meanings to molecules. What is the ‘meaning of a molecule’? It consists in its use.[20] A couple of examples should explain it. Ethanol, H3C-CH2-OH, first. There are at least a dozen standard meanings for that chemical. It provides a C2H5 ethyl group. Through prior protonation, it offers an H2O leaving group. Conversely, it is the adduct of ethylene and water. Through oxidation, it is a source of acetaldehyde. It is a fuel. It is also a hydrogen-bond donor and acceptor. It is a protic solvent. As such, it has been used from times immemorial to extract substances, such as natural products.[21] It is mildly toxic, damaging neurons and liver cells. It can be produced by fermenting sucrose-containing fruit. The H3C/H2C isotopic ratio is a signature of the place of origin of the plant, as originally detected by the SNIF-NMR method (Martin 1986, 1996).[22] The list of uses is long and open-ended.[23] Consider now cholesterol, as an example of a more elaborate, a pentacyclic molecule. It is an alcohol. It is also an ethylenic derivative. It sports a long saturated hydrocarbon side-chain. It is the prototypic molecule for the formation of so-called cholesteric liquid crystals. It serves, in the organism, as the precursor to a whole family of steroidal hormones. In turn, its biosynthesis involves the linear squalene hydrocarbon. It serves, equally importantly, as a rigidifier to cell membranes. Physicians have made it into a storybook villain, with regard to the clogging of cardiac arteries. It can be used as a scaffolding, in maintaining chemical groups at a fixed distance from one another, in order to test quantitatively Marcus theory of electron transfer (Closs 1988). And so on. How many more layers of meanings for any single molecule might one uncover (Earley 2006)? My intuition is that there is no more limit to these meanings than the unbounded imagination of scientists. Truly, any chemical becomes with time an encyclopedia of knowledge about it.[24] Each new meaning that becomes attached to a chemical species was unpredicted. Yet, after the fact, it becomes self-evident, perhaps even banal. A symptom of emergence? In contrast to closure-seeking physics (e.g., the Higgs boson), chemistry is ever-expanding. The metaphor of an ever-receding horizon (Owen 1948, Richardson 1998) has belonged to the culture of chemistry for at least a century.[25] Is there a unity to all these meanings of a molecule? Can they be subsumed into a single, umbrella-like representation? These are questions worthy of attention, needing another entire article for thorough examination. A chemist’s brain (and hands) thus stores a manifold of both concepts and percepts, regarding many a chemical. Such knowledge is akin to the mastery of a language, in both its explicit and implicit aspects (a Chomskian grammar). In its creativity as well. Is Lavoisier’s esprit de vin the same substance as, say, ethanol nowadays is to Whitesides? Has the aggregation of novel meanings over the ages modified our concepts of it, our percepts? This rhetorical question belongs, perhaps, in the same category as Nabokov’s nymphette identifying, or not, with Catullus’s Clodia. With science as tinkering, as cunningly devising ways in which to capture a protean nature in Francis Bacon’s penetrating metaphor (Hoffmann 2001), with molecular polysemy, one comes rather close to what Michael Polanyi called ‘tacit knowledge’; which I will refer to in the context of chemical diagrams and representations. In the following, I shall merely sketch out a single axis of reflection and inquiry, in line with the article I wrote a quarter of a century ago, together with Roald Hoffmann, on ‘Representation in Chemistry’. The next section, while disposing of the red herring, chemistry as a tacit science, nevertheless supports the origin of The Tacit Dimension in Polanyi’s earlier career, prior to 1933, as a research chemist. 7. Tacit knowledgeAs is well known, the philosopher Michael Polanyi started his professional life as a chemist. Prior to 1933, he worked in Fritz Haber’s Institute in Berlin. He was a co-author there together with his research coworkers Henry Eyring, Eugene P. Wigner, and H. Pelzer, on transition state theory. A full quarter of a century after his emigration to England, where his academic life resumed with a professorship at the University of Manchester, he published a book entitled Personal Knowledge (Polanyi 1958). This book introduced the notion of ‘tacit knowledge’.[26] This whole segment will make the case, obvious to a chemist but not necessarily to non-chemists, that Polanyi’s philosophical ideas were seeded in his professional experience as a chemist. To a chemist, tacit knowledge – at least implicit knowledge, this is a key distinction, lest words betray the thought – is not only an integral part of the professional life, it is also a worldview.[27] Hence, this Section will be devoted, besides this argument, to an examination in some depth of this notion. First, I shall go through an ‘Exhibit’, Singh et al. 2013, and point out some of the instances of tacit knowledge this article is interspersed with, reflecting actual laboratory practice. Second, I will relate the ‘reading’ of structural formulas, in some of its stages, to implicit knowledge. Third, I shall examine the relationship of Polanyi’s concept of tacit knowledge to Peirce’s notion of abduction. Fourth, I shall bring up the link between epistemology and iconology, based upon Erwin Panofsky’s ‘intrinsic meaning’: Is it just another name for ‘tacit knowledge’ or are they unrelated notions? A laboratory chemist constantly resorts to implicit knowledge. My first example is the indication ‘-78°’ in the ‘Experimental Part’ of a paper, such as the Exhibit (Singh et al. 2013). Any trained chemist knows to translate this seemingly sybilline instruction into ‘place the reaction flask in a constant temperature bath at -78°C, provided by dissolving dry ice, i.e., solid carbon dioxide, in acetone solution’. Indeed, this is one of several thermostated cooling mixtures, which a chemist is trained to prepare and to use. The graphic abstract to that paper (Singh et al. 2013) next includes the instruction "-78°C to r.t, 2h", whose explicit meaning is ‘remove the flask from the refrigerated bath, place it on the benchtop and proceed to let the reaction mixture slowly warm to room temperature over two hours’. Or, consider the following single sentence in that article, also written in Chemese language : "L-Proline was esterified (12) by treating it with MeOH and thionyl chloride at 0°C, followed by Boc protection of secondary amine in dry tetrahydrofuran (THF) using triethyl amine as base at rt, furnishing 13, which on LAH reduction at 0°C in dry THF provided alcohol 14." (Singh et al. 2013) The chemical recipient of this treatment is the amino acid proline, as the (natural) L-enantiomer. It can be bought from suppliers of laboratory chemicals. Its esterification means formation of an ester between its carboxylic COOH group and the simplest of alcohols, methanol (here written as MeOH), another commercial chemical, in the presence of thionyl chloride (SOCl2), also commercial. The reaction scheme bears the instruction "0°C-rt, 4h", in other words, ‘dissolve proline and thionyl chloride in methanol, held in a cooling bath, made of water with floating ice cubes, at 0°C and let this mixture return to room temperature (rt) over four hours, before extracting the desired product’. Needless to say, this totally routine series of actions is rife with implicit meanings. They all call for chemical experience, which makes the difference between a botched and a successful outcome. For instance, the stated ‘room temperature’ in fact has a meaning more elaborate than ‘the temperature in the laboratory’. It means ‘about 20°C’, hence if the actual room temperature is markedly different, one ought to switch on either heating or air-conditioning.[28] Likewise, the indication ‘four hours’ is translatable in something like ‘meanwhile, go to lunch, apply yourself to other tasks and, before you go home at the end of the afternoon, take care of this reaction mixture, so that the product be isolated, weighed and properly characterized before you leave’. I won’t belabor the point and will abstain from commenting on the rest of this one-sentence procedure, which carries quite a few other references to implicit notions. To quote Mary-Jo Nye at this point (Nye 2007): The scientific life builds upon craft skills and tacit understandings that often cannot be explained or transmitted verbally or logically. They must be learned in place, in the laboratory, the seminar, and the study. It is apprenticeship in the regimented discipline of the scientific community that serves as a demarcation between science and non-science. To sum-up the understanding reached from this look at laboratory practice, implicit knowledge consists predominantly of know-how expressed, for brevity’s sake, in a shorthand. The reason for keeping implicit (not tacit) all these pieces of knowledge is they are so well-known by practitioners to make it useless to reiterate them. At the risk of repeating myself, but to the consternation of quite a few philosophers of chemistry, who make the error of sampling it from textbooks,[29] chemistry is governed by its laboratory practice, not by its elementary teaching in the classroom (Laszlo 2013). The ‘know-how’ is crucial.[30] Thus, chemistry, as an experimental science, as the science of change,[31] thrives on know-how acquired through both experience and experimentation. It is both a science and a craft.[32] What about the adjective ‘tacit’, in the expression ‘tacit knowledge’? It derives from the Latin verb tacere (to shut up, to hold one’s speech). This is an indication all the more important in the face of assertions according to which any knowledge should be expressible as a set of logical propositions: "the possibility of possessing knowledge that cannot be wholly articulated by linguistic means emerges […] as completely unintelligible" (Johannessen 1990). The same author, though, in the same article hastens to render such an interdiction moot: "propositional knowledge, i.e., knowledge expressible by some kind of linguistic means in a propositional form, is not the only type of knowledge that is scientifically relevant." Similar conclusions can be obtained from the etymology of the pair ‘implicit’/‘explicit’. Now to chemical language, in its iconic dimension of structural formulas, pictograms, chemical equations. Their meanings, i.e., their understanding, also have a tacit side that make them difficult to master at first. Consider the three pictures in Figure 1 taken from our sample publication (Singh et al. 2013).
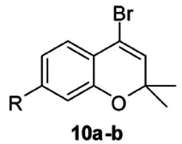
Figure 1. Formula of the reactant, i.e., starting material (from Singh et al. 2013). The starting material bears number 10. There is food for thought! In that representation of a starting material, some stuff, a white powder probably, is signified by a chemical formula – a set of markings on paper. As is very familiar, this key achievement of chemical thought, the bridging of the macroscopic (at our scale) and the nanoscopic (at the scale of molecules and atoms), started being reached during the 1860s. But it endures! Chemists have been trained to switch back-and-forth between these two length scales to such an extent that it has become a natural oscillation of the mind. Is it akin to a Gestalt switch? I will not take up this point here, it would take us too much afar.[33] Formula 10 shows, on the left, a benzene ring. This segment of the iconic information is ripe with implicit meanings. A chemist, confronted with a benzene ring, in another mental pendulum motion, spontaneously associates the alternate Kekulé formula, in which formal single bonds and double bonds have changed places. Moreover, this notion of the incompleteness of the paper formula brings up multiple other representations, such as Dewar’s, Ladenburg’s, Thiele’s, etc., which all contribute to the benzene structure and account for its aromaticity. Hence, not only does the image 10 with its benzene ring brings up all these implicit meanings, it is also pregnant with all the notions, some macroscopic (thermodynamic stability), some nanoscopic (equalization of the six bond lengths in the benzene ring), implied by aromaticity. The two rings comprised by 10 show other implicit meanings, with the elided constituting atoms. Chemists are trained in reading such formulas, mentally adding carbon atoms – six in the benzene ring for instance – and the pertinent hydrogen atoms: three carbon-hydrogen bonds, for instance, in the benzene ring of 10. Only heteroatoms are spelled out, the oxygen atom O in the ring to the right. One of the two adjacent carbon atoms bears two geminal CH3 methyl groups, that show like a letter V on its side. A bromine atom Br is another heteroatom in this formula. In other words, what to a chemist is an extremely simple molecule, carries in its shorthand representation a number of rules for translating implicit into explicit meanings. But let us consider now the middle part of the graphical abstract prepared by Dr. Panda and his coworkers (Singh et al. 2013) in Figure 2.
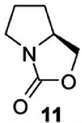
Figure 2. A portrait of L-proline (from Singh et al. 2013). It bears also a wealth of hidden meanings, nevertheless obvious to a chemist. Besides the requirement of making explicit the tetrahedral carbon atoms at the corners of the two rings and adorning them with the accompanying hydrogen atoms (three CH2 methylene groups in the top ring, one in the bottom one), stereochemical information is also provided, in the form of the wedge in bold type, which is one of the carbon-carbon bonds attaching the bottom ring to the top ring. It carries the meaning of this methyl group sticking up, being above the plane of the coplanar O=C-O atoms in the same bottom ring. We are thus dealing with a chiral molecule. But is it an enantiomer belonging to the R or S family, L or D- to use an earlier notation? The text, as already mentioned, identifies it as L-proline, one of the twenty or so natural aminoacids.
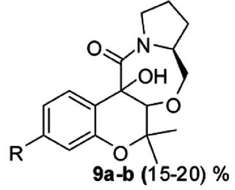
Figure 3. The achieved reaction products (from Singh et al. 2013). The adduct resulting from addition of molecules 10 and 11 is shown as structural formula 9 in Figure 3. This apparently complex formula in fact remains very simple, since the connectivity of all the atoms has remained unchanged, compared to the parent molecules: their nearest neighbors have remained the same. What strikes the chemist at the very first look at formula 9 is the poor or ambiguous sterochemical information. There are two atoms of oxygen at the junction of 10 and 11: are they both in the same half-space, either front or back? Alternatively, are they in different half-spaces, one in the front, the other in the back? One has to read the publication in order to obtain this information. In any case, whether the product 9 is a single molecule or a manifold, of up to four different molecules, its yield after isolation is nothing to brag about, only 15-20%, which is generally considered a poor result. Poor but acceptable, given that this is pharmacological work and that a useful drug may arise from those results. I have made explicit some aspects of the implicit – not tacit – knowledge of a chemist, in perusal of this particular publication. Let us now turn to the question of whether Polanyi’s tacit knowledge can be identified, or not, with Peirce’s abduction. 8. Tacit knowledge and abductionIn addition to Charles S. Peirce’s abduction being a favorite with a few chemists who have reflected on the epistemological status of their science, this mode of thought, posited Peirce as a tool of scientific discovery, has also been compared to tacit knowledge. The philosopher Phil Mullins (2002) has drawn a parallel between the two: "Just as for Peirce abduction guides and links perception and conception, for Polanyi tacit integration is at the heart of both ordinary perception and the complex theoretical conception involved in scientific discovery." Peirce argued that, besides deduction and induction, there is a third mode of inference which he called ‘hypothesis’ or ‘abduction’. He characterized abduction as reasoning "from effect to cause," and as "the operation of adopting an explanatory hypothesis" (Peirce 1992, p. 140). Abduction, not a well-defined concept of an operation of mind, has received a number of different characterizations by its progenitor, a genius who did not always provide crystal-clear expressions of his thought. Chronologically, Peirce moved from an understanding of deduction, induction, and abduction/hypotheses as three types of reasoning to understanding them as stages of inquiry very tightly connected (Rodrigues 2011). Such fuzziness of the notion of abduction has caused harsh criticism by many (Plutynski 2011). A philosopher, most helpfully, characterizes the responses to Peirce’s solution to the problem of data under-determination to a scientist as partaking either of agnosticism or fideism (Magnus 2005). To put it in a nutshell, the attraction of abduction to a chemist stems primarily from its explicit linking to diagrammatic thought, as well as to sensory perceptions: ‘chemists think with their hands’, according to a widely held dictum, which some of us believe ought to be posted over doors to laboratories. Moreover, some philosophers of science hold abduction as the dominant mode of thinking in experimental medicine, starting with its father, Claude Bernard (Debru 2010). As a discipline, experimental medicine is not at a great remove from chemistry, the two have been converging more and more during the past two centuries. Peirce (1965, p. 221) had already drawn attention to the tacit knowledge of the artisan, who, one might aver, thinks with his hands: A man can distinguish different textures of cloth by feeling: but not immediately, for he requires to move fingers over the cloth, which shows that he is obliged to compare sensations of one instant with those of another. Such perceptual realization, as the mind attunes itself to feelings from the hand, is a suitable description of abduction. It can serve in turn as the matrix model for scientific discovery, of the type chemists are wont to make. To quote Magnani (2007): concrete manipulations of the external world constitute a fundamental passage in chance discovery: by a process of manipulative abduction it is possible to build prostheses (epistemic mediators) for human minds, by interacting with external objects and representations in a constructive way. In this manner it is possible to create implicit knowledge through doing and to produce various opportunities to find, for example, anomalies and fruitful new risky perspectives. To return to the Exhibit, Singh et al. 2013, the authors sought an explanation for the poor yield of the target molecule. This observation confronted them with the unknown. In order to gain an explanation, they spontaneously resorted to the iconic language of chemistry. To frame an hypothesis they drew a picture. Thus, their recourse to both abduction, as a mode of logic, and to diagrammatic thought – both Peircean tools of intellection. They sought meaning through a picture. Which differs from Erwin Panofsky’s search of the ‘true’ or ‘intrinsic meaning’ of a picture, which he set as the aim of iconology (Panofsky 1962). Indeed, Panofsky’s iconology has been compared to Peirce’s abduction,[34] as well as to Polanyi’s tacit knowledge (Shin 1990). However, in conclusion to both Sections 7 and 8, while abduction is a productive and realistic epistemological term, the notion of tacit knowledge does not have operational value. It is too vague, ambiguous, and self-contradictory. Faced with polysemic objects, whether chemicals or their transformations, chemists are semioticians of sorts. They are interpreters who have been initiated into the art of interpretation. Where a flutist interprets a written score, a chemist interprets written diagrams. 9. The diagrammatic tool for chemical thoughtA public image of chemists is associated with the writing of formulas, involving regular hexagons.[35] Not only does this stereotype have validity, it points to an important characteristic of chemical science, its recourse to schemes and diagrams – which brings up again Peircean epistemology.[36] But first some examples. Without going further back in history, e.g., to Berzelian formulas, the structural formulas of Kekulé and others were an important step forward, in chemists’ written argumentation. Another major innovation was the electron pair theory proposed by Gilbert N. Lewis in 1916 and the octet rule appended to it by Irving Langmuir in 1919. At the beginning of the 1930s, Linus C. Pauling became to his fellow-chemists the interpreter of the new quantum physics: he resorted, for this purpose, to the iconic language of chemical formulas. His valence-bond theory was later shown to be fully equivalent to molecular orbital theory, in computation of properties such as relative energies.[37] In more recent years, numerous other diagrammatic tools were proposed and adopted by chemists. I will cite only the VSEPR representation of Gillespie-Nyholm (Gillespie 1957); Balaban’s chemical graph theory (Balaban 2013, Randic 2004); the Woodward-Hoffmann conservation of orbital symmetry rules (Woodward 1969); and E.J. Corey’s retrosynthetic analysis (Corey 1988). Molecular models, to start with that example (Francoeur 2000, Laszlo 2000), can be likened to the mock-ups of the architect. That Kekulé, the progenitor of structural formulas, as an adolescent wanted to become an architect, is an often made valid point. The analogy holds, even though architectural mock-ups are reduced in scale, whereas molecular models are (hugely) magnified in scale. What they have in common is belief of the user, in spite of the change in scale (Langland-Hassan 2011, Agler 2012). Diagrammatic thought in chemistry likewise bases its empirical efficiency on a related belief, that of calculation avoidance: one may reach a robust conclusion without recourse to a numerical calculation.[38][39] Are such beliefs logically founded, or are they only acts of faith? Coming back to molecular formulas, whether as graphs or as three-dimensional ‘objects’ such as molecular models, they are imagined entities transferred from the nanoscopic world to the macroscopic world of the laboratory.[40] Moreover, chemists are trained in the back-and-forth switch from two-dimensional paper formulas to three-dimensional molecular objects. Imagining the 3D object from the 2D formula, with its conventions – e.g., a wedge in bold type to denote a bond sticking out in the forward half-space – is an acquired skill. In addition, many chemists are capable of holding a mental image of a molecule of particular interest: not only memorizing it, also presenting it to the mind’s eye under its various faces or aspects. As anecdotal evidence of the importance of such images to chemists, we have notebooks such as Nozoe’s (Seeman 2013) in which a colleague, called upon to sign it, responds with the drawing of the formula of the molecule he or she happens to be working on. Does the formula acquire, in so doing, an obsessive, even an hallucinatory quality?[41] Indeed, the next step is sometimes materialization, turning the mental image first into an innovative construct, then going to the bench in order to bring it into the material world. Iconic thinking followed by synthesis, partial or total. What about discovery? How can one circumvent the banality of first thoughts? How does one reach an innovative thought?[42] An analogy with a craft is pertinent to convey an issue that I deem central to chemical science. Making it real, turning ideas into matter is the (relatively) easy part. The essence of this demiurgic, Promethean task of the chemist is elsewhere, upstream as it were, in forming a mental image of the coveted object. Cognitive studies have started to throw a little light on such acts of imagining. A tool for investigation is functional magnetic resonance imaging (fMRI) (Ganis 2003). Perception of symmetry is important to chemists, fMRI studies suggest that it is one of the results of the hominization process (Beck 2005, Sasaki 2005, Giannouki 2013). When the brain ‘sees’ a molecule, i.e., when we imagine a shape, the involved brain areas are very much the same as in seeing: imagining and perceiving make use of similar neurons. The overlap in the activated voxels is more pronounced in frontal and parietal regions than in temporal and occipital regions, though (Ganis 2004). Visualization has been likened, justifiably it would seem, to an occurrent belief (Langland-Hassan 2011). However, it seems unlikely for fMRI to pinpoint brain areas uniquely specialized in imagining, say, shapes of molecules (Nystrom 2000). My hunch is that, moreover, different individuals mobilize different brain areas in such tasks.[43][44] In short, emergence is totally mental, as act and process. It is helped by tools for thought (Waddington 1977), which are typically diagrammatic. 10. ConclusionI have argued in this paper that the philosophy of chemistry needs to be primarily a philosophy of action. As Bachelard eloquently kept coming back to, philosophy enriches itself from observing the actual practice of chemists. I have emphasized also their schizovision, encompassing the microscopic entities and their actual operations in the laboratory, in a constant shuttling back-and-forth. In a parallel with language, chemistry is open-ended, it is creative through the constant enrichment of chemicals with novel uses, i.e., novel meanings. I strove also to relate the non-closure of chemistry, the push of chemists for ever-receding horizons, to Polanyi’s tacit knowledge – a non-operational notion, unfortunately – and its cognate, Peirce’s abduction. Twenty years already! Hyle still going strong. Wishing for Joachim Schummer to carry on for a long time indeed! Let Alexandre Dumas have the last word, from indeed Vingt ans après: C’est une singulière chose que la pensée, et quelles révolutions un signe, un mot, une espérance, y opèrent. Notes[1] An electronic version of the 1866 reference edition, ed. by Michel Lévy Frères, Paris, can be downloaded from http://gallica.bnf.fr. [2] A neologism coined in 1950. I used it in Laszlo 1995. [3] A single example will suffice here: the molecule H3+ is predicted, without need for any calculation, to enjoy enough stability for existence from an extremely simple energy diagram (the two electrons go into a bonding state). It is drawn, in its idealization as the lowest energy state, as an equilateral triangle. [4] A recruiting device, used to lure many a graduate student to join a research group in chemistry rather than in physics, say, consists in the statement ‘you can have an idea in the morning, and have it tested in the lab by the end of the day’. Such an assertion contrasts the brief duration of a typical chemical experiment with one in modern physics, which is not unusually measured in man-years. [5] I was then (1950s) taught rudiments of glassblowing, the piercing of corks so that apparatus made of interconnected pieces of glassware would be hermetic, flame tests for various elements, etc., and never to drop a flask, however hot. During earlier years of childhood and adolescence, ‘thinking with my hands’ took the form of a miniature carpenter workbench, I successfully asked Santa Claus to bring me at the age of 5, and later on, in succession, a Meccano game, a chemistry set, and an optics set, the latter permitting construction of a microscope and a telescope. [6] Structural formulas are programmatic, they determine a line of action, a blueprint for their materialization: "le réel n’est plus que réalisation"(the real amounts only to a realization, Bachelard 1966. p. 56). [7] Luck of the draw, I refer to this publication (truly) picked at random: Singh et al., 2013. [8] An instance of science as writing (Locke 1992). Writing breaks with the time of nature (Bachelard 1993a, p. 107). The chemist-philosopher also wrote powerfully, "les opérations discursives accidentent le temps" (discursive acts break-up time with accidents, Bachelard 1993b, p. 71). [9] According to Einstein, "Common sense is the collection of prejudices acquired by age eighteen" (quoted in Bell 1952). [10] Scientists such as Max Born, Walter Hückel, Fritz London, Julius Robert Oppenheimer, Edward Teller, Eugene Wigner, and a few others. [11] Discovery of the neutron brought it to an abrupt end in 1932. [12] A referee of this paper concurred: "I agree that at times the focus on ontology within philosophy of chemistry ‘beats a dead horse’ and I also agree that the other interesting/important aspects of chemistry are often neglected." [13] If one is to believe Simon Winchester (2011), ‘to run’ has no fewer than 645 meanings: http://www.npr.org/2011/05/30/136796448/has-run-run-amok-it-has-645-meanings-so-far (consulted on 22 August 2014). [14] But how does ‘running a reaction’ translate into other languages? It is definitely not courir une réaction in French. The accepted expressions, instead, belong to the set of conduire une réaction, i.e., ‘to drive a reaction’, as one drives a car. And, for ‘starting, setting-up a reaction’, mettre en route une réaction, with likewise an automobile metaphor; exécuter une réaction, i.e., ‘to perform, to carry out a reaction’. What these various equivalents have in common with the Anglo-American phrase is the notion of performing a voluntaristic action, an act of will, that occurs in time. [15] To mention just a few others worthy of analysis: ‘trapping an intermediate’, ‘working-up a reaction mixture’, ‘sterically congested’, ‘steric bias’, etc. [16] During the late 1960s, I headed a research group in the chemistry department at Princeton. Fisher 4A zeolites served to dry the solvents we used. But what about keeping free of humidity, in Princeton’s subtropical climate, these small aluminosilicate beads? We could have bought, for hundreds if not thousands of dollars, specialized ovens sold by companies providing laboratory instruments. Instead, we purchased, for less than $50, in a local artists supplies store on Nassau Street, a little oven whose intended use was for enameling jewelry. It answered our need perfectly. [17] The graphene material occupies a field where physics, chemistry, and engineering overlap. Its preparation, which could not be simpler, repeatedly lifting a sticky piece of Scotch tape, is a lovely instance of tinkering that brought the 2010 Nobel prize in physics to the Russian scientists who devised it at the University of Manchester (Novoselov 2004). [18] Devising the PCR procedure, Kary Mullis was as much a tinkerer as a scientist (Doyle 2002). [19] Repurposing a drug is nowadays a means for the pharmaceutical industry to treat, inter alia, orphan diseases at little expense. [20] To chemists, the multiple meanings of a molecule are in the uses it can be put to, innovative uses especially. In this respect, molecules are word-like. "The meaning of a word is its use in the language" (Wittgenstein 2009, p. 43); "if we had to name anything that is the life of a sign, we should have to say that it was its use" (Wittgenstein 1958, p. 3). [21] Providing numerous after-dinner drinks. [22] This technique started Eurofins, an extremely successful multinational corporation, specialized in tracing the provenance of foods and drinks. [23] Simply Googling the name ‘ethanol’ resulted in 51.200.000 hits on 26 August 2014. [24] This is one of the reasons for the ever-increasing size of textbooks of chemistry (for factual evidence, see Laszlo 2013). They reflect, from a distance, an exponential growth over the years as also measured by the numbers of Chemical Abstracts. In view of such an explosion, the encyclopedic teaching of chemistry is condemned. Perhaps, chemistry as a discipline has reached its ‘Bourbaki moment’? It ought to be taught, henceforth, as languages are being taught, by combining total immersion with focusing on the principles only – which is what the Bourbaki group of French mathematicians started contributing to their field in the 1940s. [25] The major French chemist Georges Darzens (1912), then a recently appointed professor at the Ecole polytechnique, thus wrote: "The chemist appears to me in the guise of a traveler climbing on an endless mountain. Clouds mask the perspective. Glimpsed from afar, those trees, he fancies, are his goal; and those grandiose landscapes beyond which nothing is apparent. However, as soon as he gets there, as soon as he has traversed the fog, other horizons spring up beyond this first horizon. They are wrapped also in the same deceiving haze. And our chemist is infected with the crazy desire to progress yet further. He covets getting to a point where he alone will be left to admire the splendors now left behind him. Indeed his bold and haughty climb gives him so many wonders to look at that they enthrall him. He catches himself fantasizing about what may lay beyond and his intuition does not betray him." (My translation) [26] To quote Polanyi from a later article, "I shall reconsider human knowledge by starting from the fact that we can know more than we can tell. This fact seems obvious enough; but it is not easy to say exactly what it means." (Polanyi 1966, p. 4). [27] To Primo Levi, chemistry was an all-engulfing explanandum. He thought of it as an "indefinite cloud of future potentialities" for providing him with an inner "principle of order", for a conceptual architecture that would make the entire world legible to the self: "I would watch the buds swell in the spring" (natural history), "the mica glint in the granite" (mineralogy), "my own hands" (physiology) "and I would say to myself: ‘I will understand this, too, I will understand everything.’" (Levi 1975) [28] Selecting a reaction temperature (or temperatures) is part of establishing an operational procedure, i.e., an articulated process, a mini-history. [29] Since chemical teaching is extremely conservative, since writers of textbooks – there are a few outstanding exceptions – are not scientists working at the leading edge of chemical science, most textbooks are way behind the times. Some papers by philosophers of chemistry deal with issues such as atoms in molecules, as they were discussed in the long bygone era of molar refraction and parachor, inter alia a method to predict the densities of a range of ionic liquids from their surface tensions and vice versa. Chemistry has moved forward quite a bit since. It might be worth their while, instead of constantly rediscovering the wheel, to immerse themselves in a measure of French philosophy (Bensaude-Vincent 2005). [30] The expression ‘know-how’, in English, according to the Merriam-Webster dictionary (the OED does not mention it), refers to "knowledge of how to do something smoothly and efficiently", which is rather uninformative. Its first attestation is from 1838. Likewise, the equivalent expression in French, savoir-faire, is also of somewhat late introduction (1694, 1718), contemporary with the beginnings of the Enlightenment and its rehabilitation of the various arts and crafts, as in the Encyclopédie. The Trésor de la langue française defines it as the "easy practice of an art, a discipline, a profession, a persistent activity; manual dexterity or intellectual ease acquired through experience, apprenticeship, in a given field." ‘Acquired through experience’: the last is the keyword. Indeed, the parent Latin words are peritus (expert), experientia (experience), experiens (experimented), expertus (proven). They all derive from the vanished verb *perio(r), in turn related to the Greek word πειρα (attempt, test, trial, experience, things perceived over time). [31] According to Montaigne (Essais, bk. III, ch. II): "The world is nothing but a perennial swing. All things in it swing interminably the rocks in the Caucasus, pyramids in Egypt, both from the general swinging and from their own. Constance itself is nothing but a more languishing swinging." (My translation) [32] Chemistry and craftsmanship are close to one another. Anecdotal evidence, admittedly weak (but significant): two of the chemistry graduate students at Princeton University during the 1960s when I taught there, David MacInnes and Lou Pignolet, who in addition were roommates, made careers as college professors at Guilford College and the University of Minnesota, respectively. Both, unbeknownst to one another, have now become full-time woodcarvers. [33] Let me only remind the reader of the earlier alluded-to translation from spectroscopic data, typically nmr or mass spectrometry, to the corresponding molecule. How does one know how to give such an accurate description of the brand-new? By assembling semes, akin to the phonemes making up a word, or to syllables in a purely syllabic language such as Japanese. Such acts, on the part of the chemist, are readings – with comparable neural networks as for textual readings, predictably. They also amount to acts of naming (Kripke 1980). [34] Moreover, Peirce and Panofsky had in common the drawing of a parallel between Gothic architecture and the rhetoric of scholasticism, Peirce having anteriority in coining this seminal idea (Peirce 1871, Leja 2000, Wagner 2012). [35] A referee countered with ‘a person in a lab coat holding a flask’, indeed a traditional, stereotypic image (Schummer & Spector 2007) that, even though it no longer has any connection with reality, still impregnates advertising agencies. [36] Peirce divided iconicity into images, diagrams, and metaphors. To him, diagrammatic reasoning was a kind of experimentation, an iterative process of construction, manipulation, and observation of relationships leading to a conclusion (May 1998). [37] Gaston Bachelard’s Le Matérialisme rationnel (1953), written after a translation of Pauling’s book appeared in France in 1949, is masterly. It combines a presentation of Pauling’s theory of resonance and a commentary on its philosophical implications. [38] As Andrea Woody (2000) perceptively wrote, "In molecular orbital energy diagrams, we see how a change in representation has allowed chemistry to overcome, at least partially, the intractability of quantum mechanics in generating intelligible descriptions of molecules." [39] Which is not to say that computational chemistry lacks empirical validity. When adequately parametrized, it provides useful results. [40] Which, for Ehrlich or Pauling, extended into the biological realm (Cambrosio 1993, 2005). [41] Benign! Subjects often know that an hallucination lacks reality. However, some hallucinations are so real-like as to be disturbing (Sacks 2013). [42] A small digression is in order, here, to qualify this passage from the virtual to the real. Returning to the notion of chemists as craftsmen, I will draw on a personal experience, dating back to my youth, when I served a brief apprenticeship with the master potter Norbert Pierlot. As one holds a piece of clay on the wheel, it feels to one’s hands like a living body. The feeling is inebriating, any number of shapes may be imparted to the plastic material. However, the converse statement holds considerably more truth: most shapes that the hands give to the lump are unoriginal, totally mundane and conventional, devoid of originality. To come up with a new shape is exceedingly difficult. The social precludes and snuffs out the personal, the creative imagination is stifled by the power of internalized conventions. To create means to reject. The worn out cliché, ‘thinking out of the box’, could not be more accurate. [43] "In considering man, one fancies playing on an ordinary organ. True, he amounts to an organ, but it is a bizarre instrument, changing, variable. (Those who are able only to play on an ordinary organ) would not feel at ease on that one. Knowing where the keys are is necessary." (Pascal, Pensées, Lafuma 55 - Sellier 88 Inconstance, my translation). [44] Another analogy than that to the art of the potter is in order: the creative imagination of the chemist is akin to that of the writer of fiction or poetry. A novelist writes characters into existence. Likewise, a chemist with molecules. Where a writer will jot down doodles on the page, will attempt various sketches, will try out a paragraph or two, in description, portrayal, or narrative, the chemist will use one or several diagrams of the types referred to at the beginning of this Section. ReferencesAgler, D.W.: 2012, ‘Polanyi and Peirce on the Critical Method’, Tradition and Discovery: The Polanyi Society Periodical, 38, 13-30. Bachelard, G.: 1953, Le Matérialisme rationnel, Paris: PUF. Bachelard, G.: 1966, La Philosophie du non. Essai d’une philosophie du nouvel esprit scientifique, 4th edn., Paris: PUF. Bachelard, G.: 1993a, ‘Instant poétique et instant métaphysique’, in: L’Intuition de l’instant, Paris: Stock. Bachelard, G.: 1993b [1950], La Dialectique de la durée, 2nd edn., Paris: PUF . Balaban, A.T.: 2013, ‘Chemical Graph Theory and the Sherlock Holmes Principle’, Hyle: International Journal for Philosophy of Chemistry, 19, 107-34. Beck, D.M.; Pinsk, M.A. & Kastner, S.: 2005, ‘Symmetry perception in humans and macaques ‘, TRENDS in Cognitive Sciences, 9, 405-6. Bell, E.T.: 1952, Mathematics, Queen and Servant of the Sciences, London: Bell. Bensaude-Vincent, B.: 2005, ‘Chemistry in the French tradition of philosophy of science: Duhem, Meyerson, Metzger and Bachelard’, Studies in History and Philosophy of Science, Part A, 36, 627-49. Bwambok, D.K.; Christodouleas, D.C.; Morin, S.A.; Lange, H.; Phillips, S.T. & Whitesides, G.M.: 2014, ‘Adaptive Use of Bubble Wrap for Storing Liquid Samples and Performing Analytical Assays’, Analytical Chemistry, 86, 7478-85. Cambrosio, A.; Jacobi, D. & Keating, P.: 1993, ‘Ehrlich’s "Beautiful Pictures" and the Controversial Beginnings of Immunological Imagery ‘, Isis, 84, 662-99. Cambrosio, A.; Jacobi, D. & Keating, P.: 2005, ‘Arguing with Images: Pauling’s Theory of Antibody Formation’, Representations, 89, 94-130. Closs, G.L. & Miller, J.R.: 1988, ‘Intramolecular long-distance electron transfer in organic molecules’, Science, 240, 440-7. Corey, E.J.: 1988, ‘Retrosynthetic Thinking – Essentials and Examples’, Chemical Society Reviews, 17, 111-33. Darzens, G.: 1912, Initiation chimique, Paris: Hachette. Debru, C. & Lebrave, J.-L.: 2010, ‘"Penser avec les mains": critique génétique et épistémologie’, Genesis/Théorie: état des lieux, 148. Detienne, M. & Vernant, J.-P.: 1974, Les Ruses de l’intelligence, la Mètis des grecs, Paris: Flammarion.Doyle, R.: 2002, ‘LSDNA: Rhetoric, Consciousness Expansion, and the Emergence of Biotechnology’, Philosophy & Rhetoric, 35, 153-74. Earley, J.E.: 2005, ‘Why there is no salt in the sea ?’, Foundations of Chemistry, 7, 85-102. Earley, J.E.: 2006, ‘Chemical "Substances" That Are Not "Chemical Substances"’, Philosophy of Science, 73, 841-52. Francoeur, E.: 2000, ‘Beyond dematerialization and inscription: Does the materiality of molecular models really matter?’, Hyle: International Journal for Philosophy of Chemistry, 6, 63-84. Frontisi-Ducroux, F.: 1975, Dédale. Mythologie de l’artisan en Grèce ancienne, Paris: Maspéro. Ganis, G.; Thompson, W.L.; Mast, F.W. & Kosslyn, S.M.: 2003, ‘Visual imagery in cerebral visual dysfunction’, Neurologic Clinics of North America, 21, 631-46. Ganis, G.; Thompson, W.L.; & Kosslyn, S.M.: 2004, ‘Brain areas underlying visual mental imagery and visual perception: an fMRI study’, Cognitive Brain Research, 20, 226-41. Giannouli, V.: 2013, ‘Visual symmetry perception’, Encephalos, 50, 31-42. Gillespie, R.J. & R.S. Nyholm: 1957, ‘Inorganic Stereochemistry’, Quarterly Reviews, 11, 339-80. Johannessen, K.S.: 1990, ‘Rule Following, Intransitive Understanding and Tacit Knowledge’, Daimon, 2, 154-5. Hegel, G.F.W.: 1970 [1830], ‘Die Philosophie des Geistes’, in: E. Moldenhauer & K.M. Michel (eds.), Enzyklopädie der Philosophischen Wissenschaften im Grundrisse, Frankfurt am Main: Suhrkamp, Part III. Hoffmann, R. & Laszlo, P.: 1991, ‘Representation in chemistry’, Angewandte Chemie International Edition, 30, 1-16.Hoffmann, R. & Laszlo, P.: 2001, ‘Protean’, Angewandte Chemie International Edition, 40, 1033-6. Hoffmann, R.: 2007, ‘What might philosophy of science look like if chemists built it?’ Synthese, 155, 321-36. Houlgate, S.: 1995, ‘The Unity of Theoretical and Practical Spirit in Hegel’s Concept of Freedom’, Review of Metaphysics, 48, 859-81. Houlgate, S.: 1999, ‘Schelling’s Critique of Hegel’s "Science of Logic"’, Review of Metaphysics, 53, 99-128. Kauffman, S.A.: 1990, ‘The Sciences of Complexity and "Origins of Order"’, Proceedings of the Biennial Meeting of the Philosophy of Science Association, 299-322. Kripke, S.: 1980, Naming and Necessity, Oxford: Blackwell. Kullmann, M.: 1960, ‘Is Idealism Really Nonsense?’, Philosophy and Phenomenological Research, 20, 535-9. Langland-Hassan, P.: 2011, ‘A puzzle about visualization’, Phenomenology and the Cognitive Sciences, 10, 145-73. Laszlo, P.: 1995, La chimie nouvelle, Paris: Flammarion-Dominos. Laszlo, P.: 1999, ‘Circulation of concepts’, Foundations of Chemistry, 1, 225-38. Laszlo, P.: 2000, ‘Playing with molecular models’, Hyle: International Journal for Philosophy of Chemistry, 6, 85-97. Laszlo, P.: 2013, ‘Towards teaching chemistry as a language’, Science & Education, 22(7), 1669-1706. Leja, M.: 2000, ‘Peirce, Visuality, and Art ‘, Representations, no. 72, 97-122. Levi, P.: 1975, The Periodic Table, New York: Schocken. Lévi-Strauss, C.: 1962, La Pensée sauvage, Paris: Plon [Engl. trans as The Savage Mind, Chicago: University of Chicago Press, 1966]. Locke, D.: 1992, Science as Writing, New Haven, CT: Yale University Press. Magnani, L.: 2007, ‘Abduction and Chance Discovery in Science’, International Journal of Knowledge-Based and Intelligent Engineering, 11, 273-9. Martin, G.J., Zhang, B.L.; Naulet, N. & Martin, M.L.: 1986, ‘Deuterium transfer in the bioconversion of glucose to ethanol studied by specific labeling at the natural abundance level’, Journal of the American Chemical Society, 108, 5116-22. Martin, G.G.; Wood, R. & Martin, G.J.: 1996, ‘Detection of added beet sugar in concentrated and single strength fruit juices by deuterium nuclear magnetic resonance (SNIF-NMR method): collaborative study’, Journal of AOAC International, 79, 917-28. May, M.: 1998, ‘Images, Diagrams and Metaphors in Science and Science Education: The case of chemistry’, Almen Semiotik, 14, 77-102. Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V. & Firsov, A.A.: 2004, ‘Electric Field Effect in Atomically Thin Carbon Films’, Science, 306, 666-9. Nye, M.-J.: 2007, ‘Historical Sources of Science-as-Social-Practice: Michael Polanyi’s Berlin’, Historical Studies in the Physical and Biological Sciences, 37, 409-34. Nystrom, L.E.; Braver, T.S.; Sabb, F.W.; Delgado, M.R.; Noll, D.C. & Cohen, J.D.: 2000, ‘Working Memory for Letters, Shapes, and Locations: fMRI Evidence against Stimulus-Based Regional Organization in Human Prefrontal Cortex’, NeuroImage, 11, 424-46. Owen, E.T.: 1948, ‘The Illusion of Thought’, Journal of Philosophy, 45, 505-11. Panofsky, E.: 1962, Studies in Iconology, New York: Harper and Row. Pauling, L.C.: 1939, The Nature of the Chemical Bond, Ithaca, NY: Cornell University Press. Peirce, C.S.: 1871, ‘Review of Fraser’s "The Works of George Berkeley"’, in: Writings of Charles S. Peirce, vol. 2, Blomington: Indiana University Press, pp. 465-66. Peirce, C. S.: 1965, Collected Papers, vol. 5., Cambridge, MA: Harvard University Press. Peirce, C. S.: 1992, Reasoning and the Logic of Things: The Cambridge Conferences Lectures of 1898, Cambridge, MA: Harvard University Press. Plutynski, A.: 2011, ‘Four Problems of Abduction: A Brief History’, HOPOS: The Journal of the International Society for the History of Philosophy of Science, 1, 227-48. Polanyi, M.: 1958, Personal Knowledge: Towards a Post-Critical Philosophy, Chicago: University of Chicago Press. Polanyi, M.: 1966, ‘The Logic of Tacit Inference’, Philosophy, 41, 1-18. Randic, M.: 2004, ‘Nenad Trinajstic – Pioneer of Chemical Graph Theory’, Croatica Chemica Acta, 77, 1-15. Richardson, B. & D. Herman: 1998, ‘A Postclassical Narratology’, PMLA, 113, 288-90. Rodrigues, C.T.: 2011, ‘The Method of Scientific Discovery in Peirce’s Philosophy: Deduction, Induction, and Abduction’, Logica Universalis, 5, 127-64. Rougemont, D. de: 1936, Penser avec les mains, Paris: Albin Michel. Russell, I.W.: 1951, ‘Among the New Words’, American Speech, 26, 208-10. Sacks, O.: 2013, Hallucinations, New York: Vintage. Sasaki, Y.; Vanduffel, W.; Knutsen, T.; Tyler, C. & Tootell, R.: 2005, ‘Symmetry activates extrastriate visual cortex in human and nonhuman primates’, PNAS, 102, 3159-63. Schummer, J. & Spector, T.I.: 2007, ‘Popular Images versus Self-Images of Science: Visual Representations of Science in Clipart Cartoons and Internet Photographs’, in: B. Hüppauf & P. Weingart (eds.), Science Images and Popular Images of Science, London-New York: Routledge, pp. 69-95. Seeman, J.I.: 2013, ‘The Nozoe Autograph Books: Stories behind the Stories’, Chemical Record, 13, 483-514. Shin, U.-C.: 1990, ‘Panofsky, Polanyi, and Intrinsic Meaning’, Journal of Aesthetic Education, 24, 17-32. Singh, R.; Parai, M.K.; Mondal, S. & Panda, G.: 2013, ‘Contiguous Generation of Quaternary and Tertiary Stereocenters: One-Pot Synthesis of Chroman-Fused S-Proline Derived Chiral Oxazepinones’, Synthetic Communications, 43, 253-9. Thom, R.: 1990, ‘Vertus et dangers de l’interdisciplinarité’, in: Apologie du logos, Paris: Hachette, pp. 636-43. Waddington, C.H.: 1977, Tools for Thought: How to Understand and Apply the Latest Scientific Techniques of Problem Solving, London: Jonathan Cape. Wagner, D.: 2012, ‘Peirce, Panofsky, and the Gothic’, Transactions of the Charles S. Peirce Society, 48, 436-55. Wigner, E.P.: 1949, ‘Invariance in Physical Theory’, Proceedings of the American Physical Society, 93. Winchester, S. 2011: ‘OpEd’, New York Times, 28 May. Wittgenstein, L.: 1922, Tractatus logico-philosophicus, London: Kegan Paul. Wittgenstein, L.: 1958, The Blue and Brown Books, Oxford: Blackwell. Wittgenstein, L.: 2009, Philosophical Investigations, Oxford: Wiley-Blackwell. Woodward, R.B. & Hoffmann, R.: 1969, ‘The Conservation of Orbital Symmetry’, Angewandte Chemie International Edition, 8, 781-853. Woody, A.: 2000, ‘Putting Quantum Mechanics to Work in Chemistry: The Power of Diagrammatic Representation’, Philosophy of Science, 67, S612-S627. Pierre Laszlo: Ecole polytechnique, Palaiseau, France, and University of Liège, Belgium; ‘Cloud’s Rest’, Prades, 12320 Sénergues, France; clouds-rest@wanadoo.fr |