The Molecular Aesthetics of DiseaseThe Relationship of AIDS to the Scientific ImaginationTami I. Spector*Abstract: This paper shows how the simulated molecular forms of HIV protease allow scientists to immerse themselves in the study of AIDS while simultaneously serving their aesthetic needs. To unravel the aesthetic nature of the computationally rendered representation of HIV protease, an analysis of the aesthetic function and properties of molecules is undertaken, with particular emphasis on the properties of tension, elegance, and sublimity.
1. IntroductionAIDS, perhaps more than any other disease of this century, is a socially and visually marked disease. Laden with cultural implications, it has attracted the attention of social critics who labor to unearth its buried discourse.[1] Socially, AIDS reveals the inadequacies of biomedical science, provides a locus for disease moralists, can be carried invisibly and spread without consent, and stigmatizes particular sexual and racial communities – legitimizing prejudice. Images of AIDS link the diseased body to the deviant body and provide a mechanism for distancing the possibility of AIDS in our own lives. In the media this distancing is accomplished through the cultural lens of self-infliction, where people with AIDS are often portrayed as marginalized and isolated gay men, dark-skinned drug users, and emaciated prostitutes. Such images "construct distinctions between normal and perverse, legal and criminal, innocent and culpable, healthy and diseased" (Nelkin et al. 1991, p. 9), and feed our collective desire to stop AIDS from spreading into the mainstream psyche. Although AIDS has a variety of potential visual manifestations, including enlarged lymph nodes, wasting, and, in its late stages, paralysis, and blindness, the stigma of AIDS is most potently expressed through images of skin infections and lesions. As William Miller writes, "diseases that attack the skin in especially grotesque ways often come to be understood as allegories of the moral condition of the inside" (Miller 1997, p. 52). The particular association of AIDS with images of diseased skin is cemented in medical and media representations of the disease. In medicine, even a quick perusal through clinical textbooks on HIV infection reveals that, with the exception of sections on the dermatological manifestations of AIDS, there are few photographs of people with the disease.[2] This powerful connection between AIDS and grotesque skin disorders is captured by Douglas Crimp’s description of a priest with "his hand on the KS-lesion-covered head of a PWA [person with AIDS]– administering last rights" set at the close of the Sixty Minutes expose ‘AIDS Hits Home’ (Crimp 1992, p. 120) and by Roger Bennett’s illuminating paper on the ‘Effects of Horrific Fear Appeals on Public Attitudes Towards AIDS’ (Bennett, 1996). In this study Bennett explicitly examines the impact of public heath advertisement on attitudinal changes towards AIDS while implicitly explicating the visual connection of AIDS to diseased skin. Bennett does this by exposing the study’s participants to three different types of AIDS public health posters: a "non-emotional appeal poster" from the World Health Organization "of a man and woman walking together with hands held, above the caption AIDS kills: stick to one partner"; a "[m]id-fear" image of the "Grim Reaper figure used in the Australian anti-AIDS campaign of the early 1990s" comprised of "a line drawing of a sinister and rather frightening hooded skeleton holding a scythe, above the caption AIDS kills: Prevention is the only cure we’ve got"; and "the horrific high-fear" poster "comprised [of] four appalling pictures of physical symptoms characteristic of the later stages of AIDS: the upper part of a man’s torso covered in sores (Kaposi’s sarcoma); a man with a large chin tumor (nodal lymphoma); a swollen fungus infected tongue (candidiarsis), and a large skin abscess" with the caption "AIDS kills: don’t risk infection."In biomedical science, in contrast to the disease’s grotesque visual effects depicted in medical and media images, AIDS is encapsulated by images of the human immunodeficiency virus and computer renderings of the structure of HIV protease (Figure 1d). Thus, scientists are compelled not only by their desire to understand the pathological mechanisms of AIDS, but also by a fascination fostered by the aesthetics of their models of HIV and its molecular components. Faced with the seeming conflict between the avowed aims of science and art, how do scientists account for their aesthetic response to this especially stigmatized disease? In this paper I address this question by examining how media (Fig. 1a), medical (Fig. 1b), and biomedical (Fig. 1c) images of AIDS are mediated through the scientific rendering of HIV protease (Fig. 1d) as an aesthetic object.
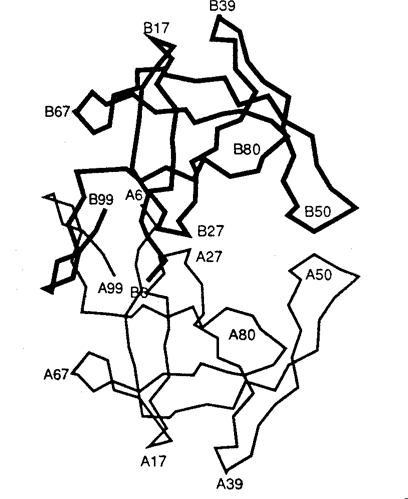
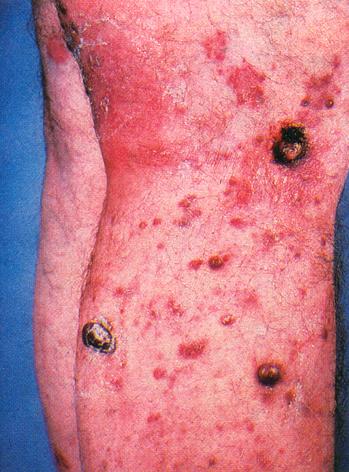 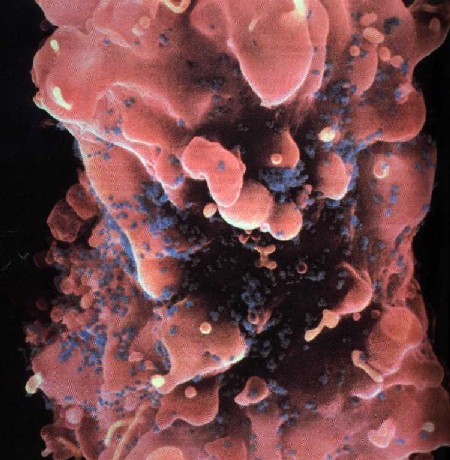  A fascinating twist on the iconization of the virus is the 2001 overview on AIDS in Nature which included an article entitled ‘Gulliver’s Travels in HIVland’ (Weiss 2001). Its publication in one of the premiere scientific journals, and therefore clearly intended for scientists, begins 2. The Inscription of HIV Protease as Scientific ObjectDelving deeper into the interior realms of scientific discourse, chemists, structural biologists, and biophysicists have taken this aesthetic transformation into the world of molecules through the structure of HIV protease. An examination of the aesthetics of this molecular world relies on understanding that representations of HIV protease are constitutive and discrete scientific entities and not simply heuristics, models, or metaphors that merely illustrate the real object. Bruno Latour’s canonical work Laboratory Life, which details how scientists constructed the peptide Thyrotropin Releasing Factor (TRF) as a neurochemical fact, can be readily applied to such representations in determining the status of molecular HIV protease as an object (Latour & Woolgar 1986).[4] Using Latour’s signifiers for constructed scientific objects we find that (1) the molecular representation of HIV protease is scientifically productive, yielding research, journal articles, and biomedical uses (i.e., it is a constitutive object); (2) there is a precise claim to the origin of its representation in time and space – that it was rendered by particular scientists on a particular date in a particular place; and (3) it is immutable to the accumulated techniques and uses applied to it since its inscription in the scientific literature (or, in scientific vernacular) ‘was discovered’.Unlike Latour’s TRF peptide, however, which exists as an object to relatively few scientists, HIV protease has meaning to most molecular scientists. But what specifically is it that has meaning as an object? Is it the representations of HIV protease or is it HIV protease itself that has objective, iconic status? That the structural representation of HIV protease is a self-contained scientific entity is highlighted by its ready distinguishability from other similar structures (i.e., other enzymatic representations). Indeed, among representations of enzymes, and even representations of other proteases, the molecular representation of HIV protease has obtained the elevated status of an instantaneously recognized cultural icon. In contrast, as a chemical substance, crystalline HIV protease is relatively indistinguishable from other crystalline solids. To provide an understanding of how images of biomolecules are constructed as constitutive objects, it is instructive to outline the experimental procedures used to generate the three-dimensional structure we know as HIV protease. This procedure began in 1985 with the determination of the "complete nucleotide sequence of the AIDS virus" obtained from the "blood pooled from several American AIDS patients" (Ratner et al. 1985, p. 280). This work, performed by collaborators at the National Cancer Institute, Harvard School of Public Health, duPont, and Centocor, revealed that the HIV virus had 9,749 DNA base pairs and that its "overall structure […] resembled that of other retroviruses" (Ratner et al. 1985, p. 280). In 1989 Paul L. Darke and co-workers at Merck Sharp and Dohme used recombinant DNA techniques to clone and express the protein HIV protease from Escherichia coli. Upon harvesting the E. Coli cell paste they isolated and characterized the protein as a 10-kiloDalton aspartic protease that is "likely to be structurally homologous with the well characterized family that includes pepsin and renin" (Darke et al. 1989, p. 2307). With the purified protein in hand scientists at Merck then used the "hanging drop vapor diffusion technique" at 4oC over four to seven days to form "tetragonal bipyrimidal crystals approximately 0.25x0.25x0.50 mm in size" of HIV protease (McKeever et al. 1989, p. 1919). These crystals were then bombarded with X-rays to yield heavy atom diffraction patterns that were subsequently read and refined to yield atomic coordinates. Finally the atomic coordinates were computationally transformed into the first molecular representation of HIV protease (Fig. 2a) (Navia et al. 1989, p. 618). Thus, through the determination of the DNA sequence of the virus, isolation methods, computational refinement, and visualization, molecular HIV has become an object laden with cultural implications for the understanding and treatment of AIDS.
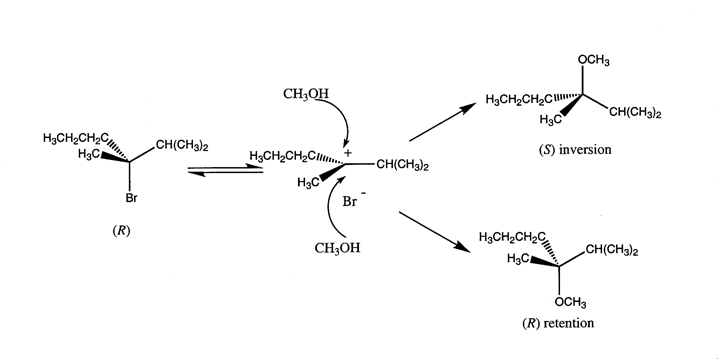
3. The Aesthetic Function and Properties of MoleculesSuch exclamations are not merely a linguistic convention. For scientists these minimalist molecular representations actually express a visual and metaphysical aesthetics which accords with the larger aesthetic epistemology of twentieth-century science. This epistemology prioritizes the capacity of the human mind to subjectively contemplate ideas beyond those that are empirically evident. An equation such as E = hn, which symbolically represents the relationship between the energy and frequency of light, and Heisenberg’s Uncertainty Principle (DpDx ³ h/2p), which encapsulates the enigma that we cannot know the precise position and momentum of a particle simultaneously, epitomize this metaphysical aesthetics. Prioritization of the abstract, along with the streamlined character of equations which express large ideas in minimalist forms, has led to our heightened perception of the aesthetic nature of quantum mechanics as captured by Heisenberg when he wrote:We now know that none of these explanations were ultimately correct, yet chemists continue to use these nineteenth-century molecular representations to postulate and communicate chemical information. The temporal robustness of these representations can be attributed to the inherent ingenuity of their form which, like a glass that can hold wine, water, or sand, may accept a plethora of theoretical contents. For chemists today these representations embody the empirically successful theories of a collective disciplinary past and provide an adaptive container for modern chemical concepts. For molecular scientists this has meant that the visual and conventional molecular structures of the nineteenth century have been imbued with the mathematical framework of chemistry developed during the early twentieth century through the paradigm of the covalent and ionic bond. Thus, rather than a bond that is a "tie which enable[s] an element […] to attach itself to one or more atoms of other elements" with "no hypothesis as to the nature of the connection" (Russell 1971, p. 90), chemists now envision bonds as a space of high electron density probability (i.e., a molecular orbital) between two atomic nuclei. Through this melding of twentieth-century theory and nineteenth-century structure, molecular representations have shifted from empirically useful pictorial heuristics to models with precise and predictive theoretical value. There are a number of aesthetic implications of this shift. Like Heisenberg’s "mathematical forms of great simplicity and beauty" modern molecular structures encode the abstractions of Schrödinger’s wave mechanics into the simplified and concise visual forms of bonds and orbitals. An example of the conceptual richness embedded in molecular forms is the scheme for an SN1 reaction presented in Figure 3. As shown here the chiral alkyl halide R-3-bromo-2, 3-dimethylhexane ionizes in methanol to form a planer, hypothetical, intermediate carbocation (i.e., a place of low electron probability or empty orbital) which is subsequently attacked by methanol’s oxygen lone-pair electrons to form a racemic mixture of products. For chemists this reaction scheme’s structures are laden with both the experimental realities of optical activity and the theoretical underpinnings of modern organic chemistry, including the dynamic nature of the reaction and the relationship between orbital theory and stereochemistry.
The conceptual superimposition of the electron onto the bond also allows chemists to imagine the dynamics of chemical transformations (i.e., the mechanisms) which have not yet been developed, rather than simply classify those that already exist. As for static structures like the Platonic hydrocarbons, the electron bond imbues mechanisms with constitutive power. For modern chemists it has become commonplace that the synthesis of a particular molecule serves merely as a vehicle for the use of an aesthetically intriguing mechanism. Organic chemists commonly use the practice of retrosynthetic analysis to imagine a path from the starting materials to the final product of a synthesis by thinking backward (i.e., from product to starting material). Step-by-step, skilled chemists apply synthetic methods and mechanistic analogues to molecular representations to conceptualize a successful synthetic strategy in reverse. Often such syntheses build towards a key mechanistic step rather than focusing on the cost efficient production of the end product.
According to Roald Hoffmann "the honesty and intensity of the aesthetic response of chemists, when they allow themselves to express it, must be taken positively, as a clue to an unformulated good, as spiritual evidence, a signpost to record, to empathize, to make connections with other aesthetic experiences" (Hoffmann 1991, p. 301). Here, Hoffmann hints at a more analytical approach to establish parameters for an aesthetics of structural chemistry. Following his lead, I have chosen to employ the concepts of aesthetic functionalism (not artistic functionalism) and the formalist terminology of art critics like Roger Fry, who articulated the interrelationship of aesthetic properties and aesthetic function in works such as Matisse’s The Conversation when he wrote that "the perfect rightness of the relations" in the painting inspires a mood of "serenity and repose" (Fry 1996, p. 115). Philosophically, aesthetic functionalists claim that one function of art is to have aesthetic properties, and that these aesthetic properties "supervene on non-aesthetic properties" (Zangwill 2001, p. 125). Thus, in Fry’s description of Matisse’s work the aesthetic property of serenity is a result of the embedment of a green, brown, red, and black rectangle in a blue background and placed next to a curved woman’s body. Simultaneously, Matisse’s painting can also have non-aesthetic functions, such as representing the nature of conversation, or even serving as the doorstop for a careless art collector.
An enhanced aesthetic response is provided by an understanding of the theoretical relations embedded in visualized molecular structures. Mark Rothko’s paintings serve as an apt example of this phenomenon in modern art. Those who look at Rothko’s color field paintings in a museum might view them as imposing and (perhaps) meditative non-representational swatches of color. For many, however, understanding Rothko’s work as a purposeful expression of spirituality within the context of the abstract expressionist movement heightens their own aesthetic response when viewing his paintings. This same principle applies to molecular appreciation with the advantage that chemists throughout the world are trained in the same implicit molecular aesthetics: they share the same taste when determining what is aesthetically pleasing, neutral, or displeasing, where taste is defined as "the ability to identify aesthetic success, to distinguish good from bad design" (Zemach 2001, p. 53) within the world of molecular representations. Aesthetic functionalism also claims that "a work (of art) must have
its origins in the intention to make a thing with certain aesthetic properties
in virtue of certain non-aesthetic properties" (Zangwill 2001, p. 137).
In chemistry this might imply that the successful synthesis of a compound
grants its aesthetic function; however, as for the Platonic hydrocarbons
the aesthetic properties of some molecules lie not only in their form but
also in the goal of making what is represented even before the synthesis
is carried out, and in the synthetic schema used to fashion them. Thus
tetrahedrane, which has never been synthesized, is an aesthetic object
with particular aesthetic properties by virtue of the intent of chemists
to synthesize the harmonious and restrained structure drawn on the page.
4. Molecular Elegance, Sublimity, and HIV ProteaseThe following list summarizes the properties I have found relevant to understanding the intrinsic aesthetic nature of molecules: tension, ephemerality, playfulness, novelty, and elegance. This is not to say that other aesthetic properties or other critical approaches to the works are not equally valid. I have chosen these because chemists themselves use formalist terminology in their aesthetic proclamations about molecules, and it is the force of this response which often appears to aesthetically motivate a chemist toward one molecular system over another. For example, almost any chemist would claim to be more aesthetically compelled by the representation of the "Mount Everest of Alicyclic Chemistry" (Paquette 1982, p. 4503), dodecahedrane, than to its open chain, plain-Jane analogue, eicosane. In the following I will focus on elegance, as this is the term most used by scientists to express their aesthetic appreciation of a particular scientific structure, be it mathematical or biomolecular.What is scientific elegance? A concise dictionary entry defines it as "ingenious simplicity, convenience, and effectiveness",[11] but a closer examination reveals that elegance is unstable in scientific discourse, at times formally beautiful, and at others psychically sublime. For scientists, elegance is situational; its meaning depends on the intent of the specific scientific community using it and the context they use it in. In the context of this paper, therefore, the more appropriate question is: what is molecular and biomolecular elegance? To answer this question I provide Donald Cram’s synthesis of "cyclobutadiene lodged in the cavity" of a hemicarcerand (Cram 1991) and W. S. Johnson’s synthesis of progesterone (Nicolaou & Sorensen 1996, p. 91) as examples (Figs. 6 & 7).
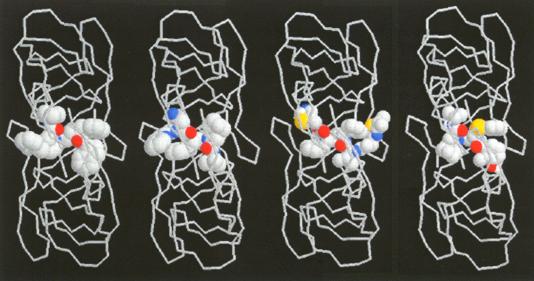
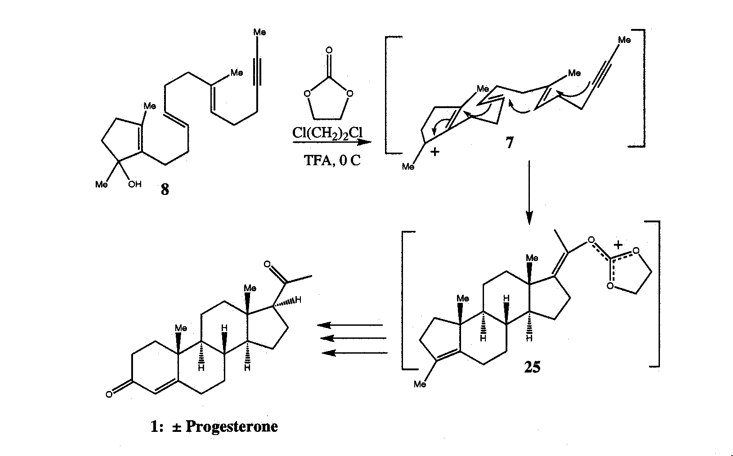 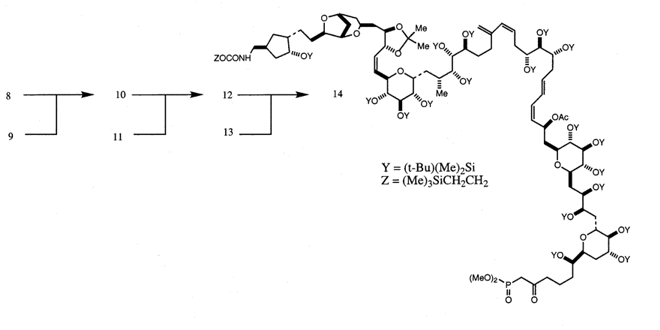 Linked by the sensation of disruption – a moment of awe expressed in astonishment – these illustrations of molecular elegance capture the transient sensation of sublimity. This sense of sublimity is expressed in Jeremy Gilbert-Rolfe’s description of the techno-sublime as characterized by "simultaneity and invisibility rather than process and moving parts, the electronic as opposed to the mechanical" (Gilbert-Rolfe, 1999, p. 80). In Johnson’s synthesis of progesterone the molecular structures encode their elegance as an instantaneous dynamic transformation of an intermediate into a complex product. In this case the existence of the product in nature is of little consequence to the chemist’s aesthetic impulse. In contrast, the elegance in renderings of biomolecules is their relationship to the natural world. These structural representations of ‘living’ molecules provide a sense of awe at our ability to comprehend the enormity and complexity of nature defined by the Kantian sublime. As described by Kant, the sublime In the late 1980s it was found that a protease enzyme was necessary for HIV viral maturation. As described earlier, this discovery quickly led to the isolation and crystallization of HIV. Upon crystallization, HIV protease revealed nature’s creation of an enzymatic machine comprised of two monomers related through an axis of symmetry. This structure is not just beautiful for its symmetry, but sublime, allowing scientists to understand and potentially conquer nature through the relationship of its form and function. The enchantment of a protein like HIV protease, as revealed by its crystal structure, is its remarkable ability to fold precisely into a molecule which performs a specific enzymatic task, and in the scientist’s ability to understand and exploit its enzymatic action by carefully examining and simulating its structure. Since its unveiling, the crystal structure of HIV protease has been used as a starting point for hundreds of experimental and computational studies. In particular, studies of the ‘flap opening’ of the enzyme encapsulate the ingenuity of nature’s design and human insight.[12] Starting from a crystal structure, the 10-nanosecond simulation shown in Figure 9 captures HIV protease in the act of curling back the tip of its glycine rich b-hairpin loop into a hydrophobic pocket to expose its active site.
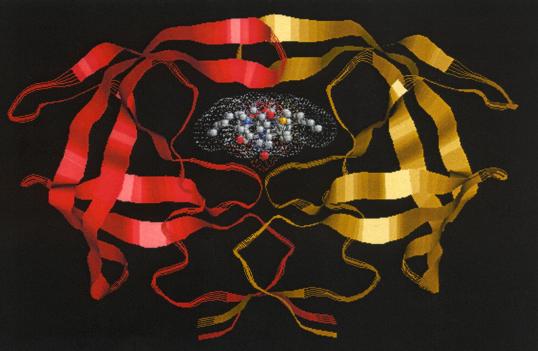
5. ConclusionAs we saw earlier in the case of cubane (Fig. 4), we can use the perspective of aesthetic functionalism to elucidate the aesthetics of HIV protease. Specifically, we can ask how HIV protease’s aesthetic properties supervene on its non-aesthetic properties so that it may express its aesthetic function. Like all molecular objects, biochemical HIV protease has significant non-aesthetic functions. These include its role as an enzyme that hydrolyzes polyproteins into proteins for viral assembly and its manifestation as an isolatable crystalline material with particular experimental properties. The representation of HIV protease also has non-aesthetic functions, including its use as a template for the design of HIV protease inhibitors. At the same time, however, such representations have the function of expressing HIV protease’s aesthetic properties through its non-aesthetic properties of symmetry and hydrophobicity, which in turn result from the formal constraints embedded in its primary amino acid sequence.It could be argued that the specific way in which HIV protease is visualized
yields its aesthetic properties. For example, it could be assumed that
what makes Roger Sayle’s image of HIV protease (Fig. 2b) aesthetically
compelling is its artistic presentation of red and gold ribbons; however,
such arguments do not account for the fact that HIV protease is more aesthetically
potent than other enzymes no matter how it is visually rendered. All students
of biochemistry learn the interrelationship of the primary, secondary,
tertiary, and quaternary structures of proteins; that the building up of
amino acids in a precise order yields a particular super-structure to these
macromolecules through a mechanism that has yet to be elucidated. At times
this mysterious collusion of intra and intermolecular forces yields aesthetically
indeterminate globular structures, while at others they produce structures
of high symmetry, like HIV protease, that scientists label as beautiful.
Formally, it is the sequence of amino acids from N-terminus to C-terminus,
and the juxtaposition of the enzyme’s secondary structures, which yields
HIV protease’s aesthetic property of balanced beauty, while its sublimity
lies in the relationship of this symmetrically dynamic form to its function
– where, like the transporting oratory described by Longinus, its sublimity
flashes "forth at the right moment [and] scatters everything before it
like a thunderbolt" (Longinus 1971, p. 77). For molecular scientists, HIV
protease transforms disorder into order by refracting its exquisite form
into a scientific object with a concise molecular structure that can be
catalogued, refined, and transmuted; a medicinal object that can be pharmacologically
exploited; and, a cultural object that absents the human form and psychologically
mollifies the chaos of the disease represented by the person with AIDS.
Notes[1] See among others: Treichler 1988, 1992 & 1999, Waldby 1996, Gilman 1988 & 1995, Leap 1990, Brandt 1991, Nelkin et al. 1991, Goldstein 1991, Sontag 1989, and Williamson 1989.[2] See among others Buckley & Gluckman 2002 and Schoub 1999. [3] This image was created for me in 1997 by E. Meng at the University of California San Francisco Computer Graphic Laboratory using MidasPlus. [4] See also Williamson 1989, Vasseleu 1991, Treichler 1992, and Waldby 1996. [5] See http://www.umass.edu/microbio/rasmol/sayle1.htm [6] See http://www.rcsb.org/pdb/molecules/pdb6_2.html, David S. Goodsell, The Scripps Research Institute, Author and Illustrator of the ‘PDB Molecule of the Month’. See also: PDB ID: 1HSG (Chen et al., 1994), PDB ID: 1HXB (Krohn et al. 1991), PDB ID: 1HXW, (Kempf et al. 1995) & PDB ID: 1OHR, (Kaldor et al. 1997). [7] See http://www.rcsb.org/pdb/molecules/pdb6_1.html. [8] See Edger Meyer’s abstract ‘An Old Look at New Structures’ from the 1999 SouthWest Macromolecular Symposium. (http://cmdnmr.tamu.edu/meyerlab/mlab/swms/pages/meyer.html). [9] See the description of the "teaching aids for macromolecular structure" (TAMS) within Eric Martz and Eric Francoeur’s ‘History of Visualization of Biological Macromolecules’, website http://www.umass.edu/microbio/rasmol/history.htm. [10] See http://www.guggenheimcollection.org/site/artist_work_md_1125.html. [11] Oxford English Dictionary Online, Oxford University Press, 2003. [12] For example see Ishima et al., 1999.
ReferencesArmstrong, R. W.; Beau, J-M.; Cheon, S. H.; Christ, W. J.; Fujioka, H.; Ham, W-H.; Hawkins, L. D.; Jin, H.; Kang, S. H.; Kishi, Y.; Martinelli, M. J.; McWhorter, W. W.; Mizuno, M.; Nakata, M.; Stutz, A. E.; Talamas, F. X.; Taniguchi, M.; Tino, J. A.; Ueda, K.; Uenishi, J-I.; White, J. B. & Yonaga, M.: 1989, ‘Total Synthesis of a Fully Protected Palytoxin Carboxylic Acid’, Journal of the American Chemical Society, 111, 7525-7530.Bakhtin, M: 1981, ‘Discourse in the Novel’, in: M. Holquist (ed.), C. Emerson & M. Holquist (trans.), The Dialogic Imagination, University of Texas Press, Austin, pp. 259-422. Bennett, R.: 1996, ‘Effects of Horrific Fear Appeals on Public Attitudes Towards AIDS’, International Journal of Advertising, 15, 183-202. Boon, M.: 1996, ‘Naming the Enemy: AIDS Research, Contagion and the Discovery of HIV’, Cultronix, 4, (eserver.org/cultronix/boon/). Brandt, A. M.: 1991, ‘AIDS and Metaphor: Toward the Social Meaning of Epidemic Disease’, in: A. Mack (ed.), In the Time of Plague, New York University Press, New York, pp. 91 -110. Buckley, R. M. & Gluckman, S. J. (eds.): 2002, HIV Infection in Primary Care, W. B. Saunders Co., Philadelphia, PA. Chen, Z.; Li, Y.; Chen, E.; Darke, P. L.; Culberson, C.; Shafer, J. A. & Kuo, L. C.: 1994, ‘Crystal Structure at 1.9A Resolution of Human Immunodeficiency Virus (HIV) II Protease Complexed with L-735, 524, an Orally Bioavailable Inhibitor of the HIV Protease’, Journal of Biological Chemistry, 269, 26344-26348. Cram, D. J.; Tanner, M. E. & Thomas, R.: 1991, ‘The Taming of Cyclobutadiene’, Angewandte Chemie International Edition English, 30, 1024-1027. Crimp, D.: 1992, ‘Portraits of People with AIDS’, in: C. Nelson & P. A. Treichler (eds.), Cultural Studies, Routledge, New York, pp. 117-133. Darke, P. L.; Leu, C-T.; Davis, L. J.; Heimbach, J. C.; Diehl, R. E.; Hill, W. S.; Dixon, R. A. F. & Sigal, I. S.: 1989, ‘Human Immunodeficiency Virus Protease’, Journal of Biological Chemistry, 264, 2307-2312. Friedman-Kein, A. E. & Ostreicher, R.: 1984, ‘Overview of Classical and Epidemic Karposi’s Sarccoma’, in: A. E. Friedman-Kein & L. J. Laubenstein (eds.), AIDS: The Epidemic of Karposi’s Sacrcoma and Opportunistic Infections, Masson Publishing, New York. Fry, R.: 1996, ‘The Grafton Gallery: An Apologia’, in: C. Reed (ed.), The Roger Fry Reader, The University of Chicago Press, Chicago. Gilbert-Rolfe, J.: 1999, Beauty and the Contemporary Sublime, Allworth Press, New York. Gilman, S. L.: 1988, ‘AIDS and Syphilis: The Iconography of Disease’, in: D. Crimp (ed.), AIDS: Cultural Analysis and Cultural Activism, The MIT Press, Cambridge, pp. 31-70. Gilman, S. L.: 1995, Picturing Health and Illness, The Johns Hopkins University Press, Baltimore. Goldstein, R: 1991, ‘The Implicated and the Immune: Responses to AIDS in the Arts and Popular Culture’, in: D. Nelkin, D. P. Willis & S. V. Parris (eds.), A Disease of Society: Cultural and Institutional Responses to AIDS, Cambridge UP, Cambridge, pp. 17-44. Heisenberg, W.: 1971, Physics and Beyond: Encounters and Conversations, Harper & Row, New York. Hoffmann, R: 1991, ‘Molecular Beauty’, Interdisciplinary Science Reviews, 16, 301-312. Ishima, R.; Freedberg, D.; Wang, Y-X.; Louis, J. M. & Torchia D. A.: 1999, ‘Flap opening and dimer–interface flexibility in the free and inhibitor-bound HIV protease, and their implications for function’, Structure, 7, 1047-1055. Kant, I.: 1951 ‘Analytic of the Sublime’, in: J. H. Bernard (trans.), Critique of Judgement, Hafner Press, New York, pp. 82-181. Kaldor, S. W.; Kalish, V. J. Davis J. F.; Shetty, B. V.; Fritz, J. E.; Appelt, K.; Burgess, J. A.; Campanale, K. M.; Chirgadze, N. Y.; Clawson, D. K.; Dressman, B. A.; Hatch, S. D.; Khalil, D. A.; Kosa, M. B.; Lubbehusen, P. P.; Muesing, M. A.; Patick, A. K.; Reich, S. H.; Su, K. S. & Tatlock, J. H.: 1997, ‘Viracept (nelfinavir mesylate, AG1343): A Potent, Orally Bioavailable Inhibitor of HIV-1 Protease’, Journal of Medicinal Chemistry,40, 3979-3985. Kempf, D. J.; Marsh, K. C. Denissen, J. F.; McDonald, E.; Vasavanonda, S.; Flentge, C. A.; Green, B. E.; Fino, L.; Park, C. H. & Kong, X. P.: 1995, ‘ABT-538 is a Potent Inhibitor of Human Immunodeficiency Virus Protease and has High Bioavailability in Humans’, Proceedings of the National Academy of Science, U.S.A., 92, 2484-2488. Krohn, A.; Redshaw, S.; Ritchie, J. C.; Graves, B. J.& Hatada, M. H.: 1991, ‘Novel Binding Mode of Highly Potent HIV-proteinase Inhibitors Incoporationg the (R)-Hydroxyethylamine Isostere’, Journal of Medicinal Chemistry, 34, 3340-3342. Latour, B & Woolgar, S.: 1986, Laboratory Life: The Construction of Scientific Facts, Princeton University Press, Princeton. Leap, W. L.: 1990, ‘Language and AIDS’, in: D. A. Feldman (ed.), Culture and AIDS, Praeger Publishers, New York, pp. 137-158. Longinus: 1971, ‘On the Sublime’, in: H. Adams (ed.), Critical Theory Since Plato, Harcourt Brace Jovanovich, Inc., New York, pp. 76-104. Maier, G.: 1988, ‘Tetrahedrane and Cyclobutadiene’, Angewandte Chemie International Edition English, 27, 1988, 309-446. McKeever, B. M.; Navia, M. A.; Fitzgerald, P. M. D.; Springer, J. P.; Leu, C-T.; Heimbach, J. C.; Herber, W. K.; Sigal, I. S. & Darke, P. L.: 1989, ‘Crystallization of the Aspartylprotease from the Human Immunodeficiency Virus, HIV-1’, Journal of Biological Chemistry, 264, 1919-1921. Miller, W.: 1997, The Anatomy of Disgust, Harvard University Press, Cambridge. Nabel, N. J.: 2001, ‘Challenges and Opportunities for Development of an AIDS Vaccine’, Nature, 410, 1002-1007. Navia, M. A.; Fitzgerald, P. M. D.; McKeever, B. M.; Leu, C-T.; Heimbach, J. C.; Herber, W. K.; Sigal, I. S.; Darke, P. L. & Springer, J. P.: 1989 ‘Three-dimensional Structure of Aspartyl Protease from Human Immunodeficiency Virus HIV-1’, Nature, 337, 615-620. Nelkin, D.; Willis, D. P. & Parris, S. V.: 1991, ‘Introduction’, in: D. Nelkin, D. P. Willis & S. V. Parris (eds.), A Disease of Society: Cultural and Institutional Responses to AIDS, Cambridge UP, Cambridge, pp. 1-16. Nicolaou K. C. & Sorensen, E. J.: 1996, Classics in Total Synthesis, VCH Publishers Inc., Weinheim. Nixon, N. & Nixon, B.: 1991, People with AIDS, David R. Godine, Boston. Nowak, M. A. & McMichael, A. J.: 1995, ‘How HIV Defeats the Immune System’, Scientific American, 273, 58-65. Nye, M. J.: 1993, From Chemical Philosophy to Theoretical Chemistry, University of California Press, Berkeley. Paquette, L. A.; Ternansky, R. J.; Balogh, D. W. & Kentgen, G.: 1982, ‘Total Synthesis of Dodecahedrane’, Journal of the American Chemical Society, 104, 5446-50. Paquette, L. A.: 1982, ‘The Chemical Transliteration of Plato’s Universe’, Proceedings of the National Academy of Science, U.S.A., 79, 4495-4500. Ratner, L.; Haseltinem, W.; Pataraca, R.; Livak, K. J.; Starcich, B.; Josephs, S. F.; Doran, E. R.; Rafalski, J. A.; Whitehorn, E. A.; Baumeister, K.; Ivanoff, L.; Petteway, S. R.; Pearson, M. L.; Lautenberger, J. A.; Papas, T. S.; Ghrayeb, J.; Chang, N. T.; Gallo, R. C. & Wong-Staal, F.: 1985, ‘Complete Nucleotide Sequence of the AIDS Virus, HTLV-III’, Nature, 313, 277-284. Rosello, M.: 1998, ‘Pictures of a Virus: Ideological Choices and the Representations of HIV’, French Cultural Studies, ix, 337-349. Russell, C. A.: 1971, The History of Valency, Humanities Press, New York. Schoub, B. D.: 1999, AIDS and HIV in Perspective: A Guide to Understanding the Virus and its Consequences, Cambridge UP, Cambridge. Scott, W. R. P. & Schiffer, C. A.: 2000, ‘Curling of Flap Tips in HIV-1 Protease as a Mechanism for Substrate Entry and Tolerance of Drug Resistance’, Structure, 8, 1259-1265. Sontag, S: 1989, AIDS and Its Metaphors, Farrar, Straus, and Giroux, New York. Tierney, R. & Mangiardi, M.: 1991, ‘Life, Death, and Hope in Angstroms: the HIV Cycle Versus BI-RG-587 Antiviral Compound’, Artforum, 29, 115-118. Treichler, P. A.: 1988, ‘Biomedical Discourses: An Epidemic of Signification’, in: D. Crimp (ed.), AIDS: Cultural Analysis and Cultural Activism, The MIT Press, Cambridge, pp. 87-108. Treichler, P. A.: 1992, ‘AIDS, HIV, and the Cultural Construction of Reality’, in: G. Herdt & S. Lindenbaum (eds.), The Time of AIDS, Sage Publications, Newbury Park, pp. 65-97. Treichler, P. A.: 1999, How to Have Theory in an Epidemic, Duke University Press, Durham. Vasseleu, C.: 1991, ‘Life Itself’, in: R. Diprose & R. Ferrel (eds.), Cartographies: Poststructuralism and the Mapping of Bodies and Spaces, Allen and Unwin, Sydney, pp. 55-64. Waddington, C. H.: 1969, Behind Appearance: A Study of the Relations Between Paintings and the Natural Sciences in this Century, MIT Press, Cambridge. Waldby, C.: 1996, AIDS and the Body Politic, Routledge, London. Weiss, R. A.: 2001, ‘Gulliver’s Travels in HIVland’, Nature, 410, 961- 967. Williamson, J.: 1989, ‘Every Virus Tells a Story: The Meanings of HIV and AIDS’, in: E. Carter & S. Watney (eds.), Taking Liberties, Serpent's Tail, London, pp. 69-80. Young, K: 1993, ‘Still Life with Corpse: Management of the Grotesque Body in Medicine’, in: K. Young (ed.), Bodylore, University of Tennessee Press, Knoxville, pp. 111-133. Zangwill, N.: 2001, ‘Aesthetic Functionalism’, in: E. Brady & J. Levinson (eds.), Aesthetic Concepts, Clarendon Press, Oxford, pp.123-148. Zemach, E.: 2001, ‘What is an Aesthetic Property?’, in: E. Brady & J. Levinson (eds.), Aesthetic Concepts, Clarendon Press, Oxford, pp. 47-60.
Tami I. Spector: University of San Francisco, Department of Chemistry, 2130 Fulton St., San Francisco, CA 94117-1080, U.S.A.; spector@usfca.edu |