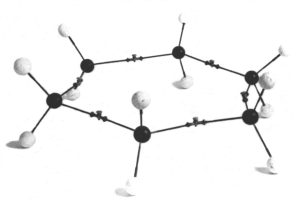
Figure 1. A structural modelm
of planar cyclohexane assembled from von Baeyer-Kekulé models. Reproduced
courtesy of O.B. Ramsay.
Abstract: Taking a critical stance towards the notions of dematerialization and inscription, this paper considers the role of physical molecular models in chemical research, specifically in the development of structural concepts and in the articulation of chemists’ knowledge of molecular structures. The main argument, illustrated through specific historical case studies, is that the materiality of these models, their specific properties as material objects, is not simply incidental to the role they have played in the development of chemistry.
Keywords: molecular models, materiality, representation, stereochemistry, conformational analysis.
Historically, the development of classical structural theory in chemistry has been concomitant with the development of non-textual, graphical techniques to render the molecular structures that structural theories, hypothesis and concepts were ostensibly about. These techniques are now so entrenched that is difficult to imagine that we could talk, write or even think about molecular structures without recourse to them. Chemists have developed and adopted over time a number of techniques and conventions to depict – to visually represent – the three-dimensional structure of molecules on a two-dimensional graphic space.[1] These allow the production of a variety of more or less elaborate structural representations which literally pepper the documents of chemists; the polysemic, yet consistent, rendering of what constitutes for all practical purposes a molecule’s identity.[2]
Another related approach commonly adopted for the representation of molecular structures in chemistry has been that of physical molecular modeling, i.e., the representation, or rendering, of molecular structures through actual physical structures, commonly referred to as molecular modelsm[3], or less commonly as physical modelsm or structural modelsm. This approach has been a constitutive practice of modern chemistry (Francoeur 1997) and its origin can be traced as far back as its graphical counterpart (Ramsay 1974a; Ramsay 1974b). The practice of physical molecular modelingm is basically rather simple. It is the production and use of three-dimensional structures that render, more or less to scale, the spatial position of atoms (or groups of atoms) in a molecular structure, as well as the bonds between them. Most molecular modelsm are assembled from commercially available modeling kits, which generally consist of modular elements (atomic units, or species, and connectors) that allow a vast number of different structures to be assembled. Although a seemingly simple task, the practice of molecular modelingm has led to the proliferation of conventions and tools.[4]
Recently, Pierre Laszlo (1998), considering specifically the practice of chemical analysis, discussed the conversion of a specific chemical sample, through instrumentalized readings, into a "molecular object", an object which can be made ostensible using one of the conventions discussed above. He described this process of translation as one of dematerialization, concluding that the work of analytical chemists consists mostly of handling mental representations, and that chemistry is to a great extent a "science of mind".[5] This process described here by Laszlo, by which the chemists’ proximal concern becomes detached from the object of chemical analysis (the sample), falls generally within current science studies accounts of instrument-mediated observations in the experimental sciences. On the other hand, underlying his concept of dematerialization is the idea that the representational techniques and conventions of chemistry are no more than a poor reflection of the abstract mental objects which constitute the true and legitimate knowledge of chemistry, crude but necessary, if only for the purpose of communication. The material signs of the chemists are to be considered (following Laszlo’s metaphoric use of language, or rather parole) as no more than a mere supplement, "a storage medium to assist memory inessential to the meaning of language" (Lenoir 1998, p. 5). This position clashes somewhat with current theoretical movements within philosophy, literary studies and science studies which emphasize the materiality of literary and scientific inscriptions, upholding the idea that writing (and inscribing more generally) is "constitutive of meaning rather than a passive medium for restoring the presence of language to thought" (idem.). My aim here is not to resolve this tension. I simply want to suggest that what can be understood in the broadest sense as the materiality of the signs used by chemists in the course of their work does somehow, in some circumstances, matter.
The circumstances I have in mind here are more closely associated with the forefront of chemical research, the development of new concepts and theories, than with routine, entrenched practices such as chemical analysis. Already, Klein (1997; 1999) has made an interesting and convincing argument in the case of Berzelian chemical formulas and the development of the concept of substitution in the 19th century. Developing the notions of epistemic techniques and paper-tools, she has shown how these Berzelian formulas, as used by the likes of Dumas, were not a simple repository for established knowledge. She argues, for example, that in relation to the development of the concept of substitution, these "chemical formulas were not only the direct referents of the new conception, but also the paper-tools by which it was produced" (Klein 1997, p. 43).
My own goal here is to illustrate and explore how, concretely, from the early days of stereochemistry, chemists have used molecular modelsm in various research settings and projects; how, in a literal sense, the use of modelsm was an integral part of the articulation of the chemists’ knowledge and experience of molecular structures, as conceptual entities. I make no claims of being exhaustive or systematic in my treatment of this topic, and my case is restricted to organic chemistry. Nevertheless, the examples I offer here will be sufficient, I believe, to show how in some sense the development and extension of what could be called "structural thinking" in chemistry has hinged at specific times upon the working out and the sorting out of the geometrical and mechanical properties of molecular modelsm.[6]
Before going any further, let me go back briefly to the "materiality of text and inscription" approach which I discussed briefly above. The concept of inscription is at the heart of this approach in which scientific research is considered in terms of a quasi-literary practice and which focuses on the graphematic materiality of scientific representational devices; a trend which implies a move from representation in scientific practice to representation as scientific practice (Rheinberger 1997). Bruno Latour (1986, 1990, 1993a) has probably been one of the most influential authors in the development of this approach. The process of scientific knowledge production (or rather, the condition of possibility for such production)[7] presented in Latour’s work is invariably one of transition from ‘things-in-themselves’ to a semiological realm; a passage from raw materiality to a graphematic space which allows the full deployment of the "powerful functionalities"[8] of inscriptions[9]. In other words, Latour equates the epistemic dominion over the world with the graphematic two-dimensional space of inscriptions, while the tangibly three-dimensional is explicitly associated with perceptual and epistemic "anomie".[10]
The graphematic ‘condensation’ of chemists’ experience and knowledge of matter in the form of graphical representation of molecular structures falls neatly into the framework elaborated by Latour and other proponents of the ‘semiotic turn’.[11] But their notion of materiality, grounded in the concept of inscription, is of little help when considering the use of molecular modelsm in chemical research. In the cases I will be discussing here, we will be looking at scientists involved in the articulation of ideas, hypotheses, traces, diagrams or theoretical statements, not through further graphematic translation, but through their translation into synthetic objects – structural modelsm – which become the tool and the means of this articulation. From a Latourian perspective, we are facing a peculiar phenomenon, i.e. cases where physical objects become the privileged mode of access to ‘epistemic things’ at points far downstream from where we expect scientists to have traded ‘things-in-themselves’ for signs and symbols – for inscriptions.[12] Let me consider this point further.
First of all, as I have argued elsewhere (Francoeur 1997), physical modelsm in chemistry do not constitute a sudden about-face on the Latourian path that leads from complex three-dimensional objects to simple, less confusing two-dimensional ones, a return to the ‘objects’ scientists left behind by translating them into graphs, diagrams and similar inscriptions. Relative to events and phenomena recorded in the lab, physical and graphical representations of molecular structures are equally ‘eidetic’. Molecular structures, and a fortiori any means of representing them, are arguably not about what molecules ‘look like’.[13] To use a distinction made by Galison (1997), any representation of molecular structure does not stand in a homomorphic relation to a referent ‘out-there’, but only to other structural representations. They should rather be considered as ‘synthetic’ representations, or interpretations, that stand in a homologic relation to empirical events and experimental phenomena – events and phenomena which they can be construed as either explaining or predicting. In other words, and to paraphrase Michael Lynch (1991, p. 208), there is no such thing as comparing the structural representation of a molecule to the ‘real’ thing, since it is through representational work that a molecular structure becomes coherently visible. The realm of molecular structures is thus essentially cultural, i.e. coextensive with the means chemists have given themselves to show, talk about and work with these structures – means which are, ceteris paribus, epistemically equivalent[14] while phenomenologically distinct.
Yet, it seems difficult to shake the idea that three-dimensional physical, modelsm are somehow more ‘real’ than their graphical counterparts. For example, in his discussion of graphical methods of representation in engineering, Ferguson claims that the advantage of modelsm is that they "can take an observer one step closer to reality than can a drawing [...]". This position appears, at first glance, to contradict the argument of epistemical equivalence. On the other hand, Ferguson’s notion of "reality" is in fact more phenomenological than epistemological:
Let me now move on and illustrate these points by discussing specific
uses of modelsm in chemical research, using
examples taken from the development of stereochemistry and conformational
analysis.
In 1885, Adolf von Baeyer reported his ‘strain theory’ of atomic bonds in cyclic compounds (1885). His argument ran roughly like this: given the model of the tetrahedral carbon atom proposed by van’t Hoff, any cyclic compound composed of fewer or more than 5 atoms will see its valency forces deviate from its ideal orientation. Carbon bonds in these compounds are thus ‘bent’, or ‘strained’. On this basis, von Baeyer proposed a structure for cyclohexane that was both planar and strained. This proposition has of course puzzled later chemists for whom molecular modelsmc self-evidently imply the non-planarity of cyclohexane (Eliel 1975). O.B. Ramsay (1981) has suggested in particular that since von Baeyer had demonstrated experimentally the relationship between benzene and cyclohexane, he must have felt compelled to extend the planar, hexagonal shape of benzene to cyclohexane. Furthermore, von Baeyer indicated in his article that the sense of his proposition could be made evident by using "Kekulé ball models" (Kekulé’sche Kugelmodelle), an early type of ball-and-stick modelsm. These had been designed by Kekulé in the 1860s, thus before the advent of stereochemistry. Their tetrahedral arrangement provided a convenient way of assembling models of double and triple bonded structures and held no further signification for Kekulé. A particularity of these modelsm, as Ramsay has pointed out, is that the bond between two carbon atom models is made up of two wires (representing valency forces), each coming out of its respective carbon model, joined in their middle by a flexible joint.[16] This arrangement was quite different from later standard models where the shared covalent bond is represented by a single wire between two atoms, and it does make the strained planar structure consistent with the assumptions built in the modelsm that von Baeyer used (Figure 1). What exact role these models played in the initial formulation of the concept of strained, planar, cyclic molecules is not clear, but, as Ramsay concluded, its rationale can be better understood by careful consideration of both von Baeyer’s writings on the topic and the modelsm he used (Ramsay 1975).

Figure 1. A structural modelm
of planar cyclohexane assembled from von Baeyer-Kekulé models. Reproduced
courtesy of O.B. Ramsay.
Five years later, another German chemist, Hermann Sachse, suggested there was no need to postulate a strained structure for cyclohexane, stating instead that two non-planar, strain free conformations[17] were possible (1890). These conformations, which he called unsymmetric and symmetric, were respectively similar to the boat and chair forms familiar to later chemists. No illustrations of these conformations were given in his article. Sachse did however instruct his readers how to assemble modelsm of these two forms, using cardboard tetrahedra attached to octahedral shapes. This particular choice of models seems to have been dictated by the way they related to his subsequently published mathematical argument (Sachse 1892). We thus see in both the cases of von Bayer and Sachse a reliance on modelsm, rather than simple images, to illustrate and demonstrate their respective (and antithetical) structural concepts. Sachse goes further in discussing the role of modelsm. In the course of his argument, Sachse suggests that one of the conformations he proposed, the asymmetric (or boat) conformation has some flexibility and that it could change its shape without any deviation (Abweichung) of the bond angles. He explains that this phenomenon was suggested by his calculations but confirmed through experiments (Versuche) with Kekulé ball modelsm. We thus see here clearly how modelsm come to define and embody a phenomenon (today referred to ‘twist conformers’). That phenomenon is, regardless of whether it is/was true of actual molecules, witnessably and demonstrably true of certain types of modelsm; an unintended emerging property stemming out of intentional or unintentional conditions (constraints and degrees of freedom) built into these modelsm.
It was to be decades before the conformations proposed by Sachse were empirically verified and accepted. Von Baeyer’s planar cyclohexane remained dominant in organic chemistry well into the 20th century (Russell 1975). Sachse had recognized and discussed the possibility of interconversions between the strainless conformations he had proposed, but believed that they did not happen under regular conditions, especially since these interconversions involved a complete or partial passage through the strained conformation defined by von Baeyer. This led to the prediction of a number of isomers whose existence could not be confirmed. Nearly 30 years later, Ernst Mohr, professor of chemistry at Heidelberg, elaborated on Sachse’s hypothesis (Mohr 1918). Mohr suggested in particular that the interconversion, or version, of conformations in cyclohexane happened constantly at room temperature, which, if true, made experimental verification of Sachse’s hypothesis impossible.
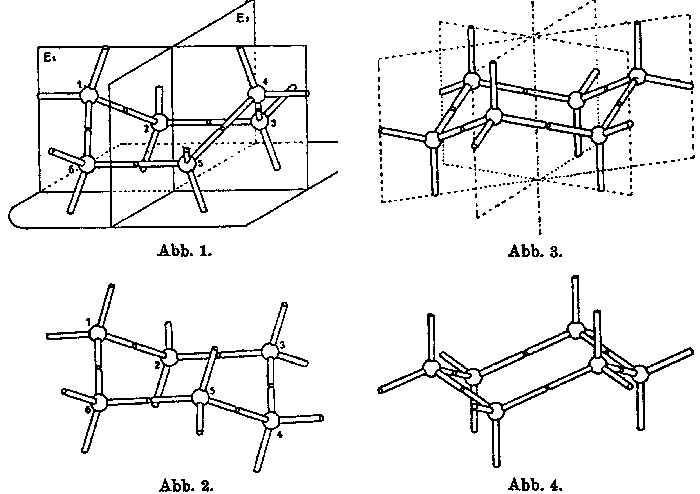
Figure 2. Figures (Abbildungen) 1 to 4 from Mohr’s 1918
article.
Mohr’s illustrations and language in this article make it clear that he is not considering the configuration of disembodied structures, but rather of structures as embodied in modelsm (whose origins and characteristics he does not otherwise discuss). This is particularly striking in his discussion of the phenomenon of interconversion (Figure 2). He thus instructs his readers, for example, that "one can transform the trigonal model (Abb. 3) into its mirror image model (Abb. 4), by turning up the atom models directed downward and turning down the atom models directed upwards. Through a still simpler alteration of shape, the rhombic model can be changed into the trigonal model (and vice versa): turn down the 4th carbon atom in Abb. 1 with a simultaneous rotation of the 3rd and 5th atom, so as to obtain Figure 2, which is the trigonal model in a somewhat different orientation than depicted in Figures 3 and 4" (p. 317, my translation). Mohr is obviously inviting his readers to virtually witness manipulation performed on modelsm, manipulations which they could repeat should they doubt the description, or should they simply wish get a better understanding of the phenomena. Like Sachse, Mohr reports experiments performed with modelsm. Thus, in his discussion of fused double ring structures, he tells us that "As experiments with models show one can construct a fairly large number of such combinations of atoms in a strain-free form. They are without exception spatial constructions; the centers of their carbon atoms do not lie on a plane […]" (p. 321, my translation). On this basis, Mohr postulated two isomers (cis and trans) for decahydronaphtalin which are not interconvertible, since the bond that forms the shared side of the two rings locks the conformations in place.[18] In other words, interconversion cannot take place without breaking that bond.
Seven years later, Wilfred A. Wightman, chemist at the University of Leeds, offered again a modeling study of the cyclohexane structure (1925). Summing up the postulates previously proposed by Sachse and Mohr, he pointed out that "the principal objection to the acceptance of Sachse’s structures as stable configurations is the large number of isomerides they would require" (p. 1422). The boat structures (which Wightman called type II), in particular, would require the existence of several monosubstituted cyclohexanes. Nevertheless, he continues by suggesting that "it is of interest […] to determine whether co-ordinated relative rotations about the single bonds are possible without strain and to examine the consequences of such a phenomenon; to this end, the author has constructed models in which these mechanical requirements are fulfilled" (p. 1423).
Wightman provided no detailed description of the modelsm he used,[19] although from the illustrations accompanying his text, it seems quite likely that they were very similar to the modelsm used by Sachse and Mohr (i.e. a variation of the ball-and-stick motif). Not surprisingly, perhaps, he repeated the observation that while the chair form of the cyclohexane structure is rigid, the boat form is "loose", i.e. that it can without strain take on different conformations, a situation which, he concludes, "annihilate[s] the isomeric possibilities above mentioned" (idem.). On the other hand, he does not mention that these observations had already been reported by both Sachse and Mohr, a fact he might have conveniently ignored to give more relevance to his own results.[20]
Wightman furthermore concluded that investigations with modelsm indeed "confirmed" the theoretical existence of a cis and trans form of decahydronaphtalene, as postulated by Mohr. The paper nevertheless ends with a word of caution, spurred by a recent announcement that a third isomer of decahydronaphtalene had been isolated: "It has therefore seemed desirable to publish the above detailed examination of the isomeric possibilities of this substance, in view of the probability that the existence of more than two isomerides will lead to the abandonment of the Mohr postulate" (p. 1424).
In these briefly described cases, we get a sense of how molecular modelsm, as analogic devices, became proximal objects of investigations, investigations which sought to explore and determine the geometrical and mechanical properties of the structure they embodied. Through these were postulated and demonstrated envelopes of theoretically possible configurations and their attendant conformations. In other words, modelsm were an intrinsic part of the definition of these possible conformations, not simply a means of illustrating them.
The key question was of course whether the knowledge derived from considering the reality of modelsm was true of molecular substances, or indeed of any relevance at all, and if so, to what extent? The empirical verification of these structural postulates remained for many years problematic. Evidence from test tubes was not always forthcoming, and if it was, it rarely proved unambiguous and decisive. Yet, whether they were considered viable working hypotheses or misleading speculations, there is little doubt that by the 1920s, the structural phenomena investigated, experienced, and demonstrated through molecular modelsm were becoming an integral part of chemistry’s conceptual landscape.
The fact that the conformations predicted by modelsm could actually be considered in agreement with experimental results was sometimes the object of surprise. In 1922, the Dutch chemist H.G. Derx (1922) published an investigation of the conformations of cyclopentane, -hexane, and -heptane based on the boric acid method. In this article, he considers strain-free versions of these structures and their various possible conformations (or arrangements, as he called them) as derived from simple skeletal modelsm, photographs of which can be found in his text. According to Derx, these modelsm were essential in order to visualize and demonstrate what he called the phenomena of rigidity and flexibility inherent to such structures, phenomena which he considered otherwise almost impossible to describe. He concluded that the conformations derived from the modelsm agreed with the experimental results, a fact which he described as "even more surprising if one takes into consideration that these models are but a crude imitation of the real relations that exist within the structure of a molecule" (p. 315, my translation).
It is important to note that the rigidities and flexibilities denoted here by Derx are not so much general properties of the structures that he considered, but rather properties of these structures as assembled from a particular type of modelsm. Choices made regarding the type of modelsm to be used in structural investigations, thus of the properties exhibited, could have clear influence on the way hunches and hypotheses were pursued. This comes across clearly, for example, in a paper by American chemists Carl Marvel and C.A. Glass on monosubstituted cyclononane (1938). These investigators explore the hypothesis that a monosubstituted cyclononane might exist in two optically active forms. They report that investigations with space-filling modelsm of the Stuart type confirmed the possible existence of enantiomers, which prompted them to synthesize and resolve the substance. No evidence of resolution was obtained, which led to the conclusion that the structure of monosubstituted cyclononane was less stable than what the model had let on. This negative result is turned into a lesson on how to interpret specific aspects of space-filling modelsm:
Conformational analysis, by introducing structural considerations into areas where they had been hitherto absent,[26] made molecular modelsm much more relevant to most organic chemists than they had been till then. Now much could be understood and explained by the simple inspection of carefully assembled modelsm. Suggesting that polar (axial) bonds in cyclohexane are more hindered than equatorial bonds, Barton would, for example, indicate that "an inspection of models makes this reasonable for a polar bond is always close in space to two other polar bonds each attached to the next but one carbon atom, whereas there is no similar relationship for equatorial bonds" (Barton 1950, p. 318). It is quite likely that a common use of modelsm was for such evaluation of the relative spatial proximity of groups or atoms in attempts to understand, explain, or predict specific reactions.[27]
Beyond the qualitative inspection of structures and conformations, modelsm were also used to derive structural variables important to both theoretical work and the interpretation of physical measurements. Thus, when he established the preferred conformations of decalin and cyclohexane by calculating the interaction energy of non-bonded atoms in various conformations, Barton used equations which required that the distance between the nuclei of every possible non-bonded atom pair be known (Barton 1948). In order to obtain these distances, Barton simply made the measurements with the help of a ruler on skeletal modelsm he had specially designed for that purpose (Barton, personal communication). Barton did not discuss these particular modelsm in his early papers on conformational analysis. He did make extensive use of them in talks and lectures, which led to exposure and numerous requests for information (Barton, personal communication). There are indications that, through informal channels, they had started to be adopted in laboratories for tasks such as the study of conformations (Braude and Sondheimer 1955) and the calculation of dipole moments (Nace and Turner 1953). In 1956, Barton published a description of the modelsm, with details about their construction (Barton 1956). In this publication, Barton discussed the use of the modelsm briefly, stating simply that "the accuracy of manufacture and scale of these models is such that quite satisfactory measurements with a metre rule can be made of the distance between atomic centres" (p. 1137).
Among the explicitly reported uses[28] of the Barton modelsm in the next decade there are, as mentioned above, the calculation of dipole moments (Nace & Turner 1960), the calculation of interaction energies (Barton et al. 1960), the calculation of diamagnetic shielding (Lenz & Heeschen 1961), and the calculation of coupling constants (Cross 1964; Lemieux 1961). Most reported uses revolved around measurement of structural variables such as interatomic distances and dihedral angles between neighboring molecular groups. In a number of papers, modelsm are simply used to obtain dihedral angle values which are then compared to nuclear magnetic resonance measurements performed on molecular substances (Coxon & Hall 1964; Hall 1964; Perlin 1964). In other words, modelsm were not in those circumstances simply a conventional means for chemists to represent the molecular objectsc ‘behind’ the instrumental inscriptions, but part and parcel of the process of giving meaning to these inscriptions in the first place, part of the possibility of interpreting these inscriptions as signifiers for these molecular objectsc.
Measurements performed on modelsm were not always considered a simple affair and were done (and reported) with the care one would expect in the case of empirical manipulation, to avoid possible errors. Thus, Cross reports the following method for measuring the distance (r) between two specific atoms in 26 different steroid structures:
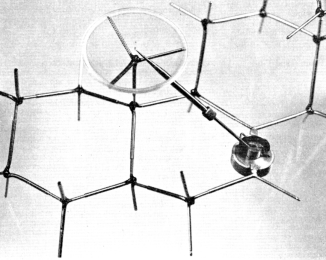
Figure 3. One of the device designed by J.W. ApSimon and his colleagues to measure angle and distance between two groups in the modelm of a molecular structure. Reproduced courtesy of J.W. ApSimon.
First, historically, the investigations of specific molecular structures through modelsm has been a mean of revealing the mechanical properties of these structures as physical objects in their own right, properties which emerged out of explicit and implicit choices made in the course of their production. These properties could then be ascribed, tentatively, to the molecules these material structures stood for, in an analogical abduction process which sometimes proved not only successful but also enlightening (while it could, of course, sometimes prove misleading). It seems clear, for example, that modelsm did not become post hoc a clever and convenient means of illustrating preexisting concepts such as the interconversion in cyclohexane, concepts which would have been derived simply from cogitation on mental representation.[34] In other words, the production of concepts can involve at its core not only the ‘handling’ of mental representation, but also the handling of material representations. If the word dematerialization has any use here, it does not so much describe a formal property of chemical analytical processes, but rather this tendency, rather common in the formal chemical literature, to treat work with modelsm as if it never happened.[35]
Second, we also have seen how modelsm have been used as devices to ‘survey’ molecular structures, to gain qualitative and quantitative knowledge about the spatial arrangements of atoms and groups in these structures. This point brings up two perspectives on models. On the one hand, the model can be seen as a ‘map’, or a quasi-inscription, the result of a long chain of re-representation that extends much further back than the local site of use,[36] an object that shows relative gain in universality, standardization and capacity to circulate. Yet, they worked precisely because in many ways they were exactly the inverse of what is explicitly defined as inscription. On the other hand, there is the model as a quasi-specimen, raw ‘matière à investigation’. In laboratory settings, as the cases I have discussed thus far show, modelsm are not simply observed. They are submitted to various manipulations, assembled, probed, and measured. In other words, the qualitative and quantitative properties of the modelsc are not always a given on which scientific investigations could rest. They often need to be worked out and sorted out in the process of performing these investigations, and here, as we saw, modelsm sometimes proved useful.
Simply attending to the materiality of modelsm
in the course of their deployment in chemical research, as I have done
here, certainly does not constitute a complete account of the many interwoven
practical and epistemological problems at play in molecular modelingmcas
a research practice, nor does it provide more than a glimpse into the development
of chemistry’s increasingly complex understanding of molecular structures.
Yet, it does compel us to consider that such devices, at the fulcrum of
the articulation of theories and concepts, are also what chemistry has
been about.
[2] On this, see particularly Luisi & Thomas 1990, p. 68.
[3] In order to avoid any semantic confusion, I will use the formulation ‘modelm’ whenever the term model is used in this text to refer to actual material objects, such as the molecular models discussed here. Accordingly, I will use ‘modelc’ for models that can be thought of as concepts or theories. This will help reduce the confusion that can arise when describing events where modelsm are used to represent a modelc, or when modelsm are used in the process of working out a modelc.
[4] Judging from the available technical surveys (Smith 1960; Walton 1978), it is possible to roughly estimate that dozens of different types and varieties of modeling systems are (or have been) available on the market and that as many different types of modeling components have been designed and manufactured by particular laboratories and institutions to suit specific needs. This reflects the wide variety of needs and purposes chemists face in their exploration of molecular structures.
[5] Although Laszlo considers here ostensibly the practice of analytical chemistry, the formulation of this latter conclusion might be interpreted as applying to the whole of chemistry.
[6] I need to point out that I am interested here strictly in the relationship between a specific category of representational devices and the genesis of specific concepts and theories. The ultimate epistemological status of these concepts and theories is actually of very little relevance to my argument.
[7] Latour claims, for example, that the specificity of our modern scientific culture can be accounted for in terms of the development of writing and imaging craftsmanship, i.e. "these small, unexpected and practical sets of skills to produce images and read and write about them", plus the means to make them reproducible and mobile (1990, p. 22).
[8] I am borrowing this expression from Rheinberger 1994.
[9] Two-dimensionality and the capacity to accompany text are of course but two of the much touted attributes of inscriptions, which also include mobility and immutability, as well as the possibility, through perspective, of dominating three-dimensional objects ‘out there’ with paper, pen, and ruler.
[10] Thus, Latour states clearly that "in the debates around perception, what is always forgotten is this simple drift from watching confusing three-dimensional objects, to inspecting two-dimensional images which have been made less confusing"(1990, p. 39).
[11] For a discussion of the notion of semiotic turn in science studies, see Lenoir 1998.
[12] Latour would certainly not deny that things can act as signs, but if they do, it is as ‘proto-inscriptions’, i.e. objects that share some, but not all, the attributes of inscriptions. They can be nothing more than intermediary steps in the process of reduction/amplification. This comes across quite clearly in Latour 1993b.
[13] In fact, any argument as to what molecules ‘really’ look like is bound to be technically moot, since, as a prominent biophysicist noted, "for something smaller than the wavelength of light, there is no such thing as showing how it really looks on the molecular level" (Richardson et al. 1992, p. 1186).
[14] By epistemical equivalence, I mean that these various representations stand at an equal distance from what is classically understood as empirical data. On the other hand, they might embody or highlight various possible interpretations of these same data.
[15] I am indebted to Ursula Klein for the explicit formulation of this point.
[16] These modelsm would later be known as Kekulé-von Baeyer models and remain available commercially well into the 20th century (Ramsay 1974b).
[17] Sachse used the term Konfiguration.
[18] Mohr would also have used modelsm to explore the various possible strain-free configurations of decahydronaphtalene derivatives; see Mohr 1922.
[19] These were obviously not commercially available modelsm, as Wightman acknowledges the help of a certain Neville Warr for their design and construction.
[20] Alternatively, one could surmise that he had only second-hand knowledge of Sachse’s and Mohr’s general postulates and no knowledge of the particular details of their work and argument.
[21] Following the different meanings of the term abuser in French, one could see the abuse occurring at different levels. One could say, for example, that the investigators unwillingly committed abuse by unjustifiably overstretching the analogy between the modelm and the molecule under investigations. Alternatively, it could be said that the modelm abused, i.e. misled, the investigators.
[22] This cautionary tale, although far from being unique (Francoeur 1997, p. 30), is certainly peculiar in that it is the only one I know of that has been published in the formal research literature.
[23] From Leybold Didactic GmbH, Hürth, Germany.
[24] However it is often pointed out that most users will find it extremely difficult to distinguish between these two conformations in such space-filling modelsm.
[25] For a more complete treatment of the early history of conformational analysis, see Ramsay 1981.
[26] As Barton would candidly put it, "now, everybody looks at everything carefully in three dimensions. When I was a student, nobody even bothered" (quoted in Borman et al. 1998, p. 40).
[27] One clear illustration of the use of modelsm in ‘eyeballing’ the relative spatial proximity of atomic groups is provided by Freifelder (1965), who reports using space-filling models to investigate the contact between the surface of a catalyst and a molecular group to undergo reduction, so as to understand the rate of that reaction in the case of specific structures. The basic technique could not be simpler: build a model of the molecule of interest, place it against a plane surface (which stands for the catalyst). If there is a noticeable gap between the surface and the molecular group, reduction is likely to be more difficult to achieve.
[28] These have been traced through the Science Citation Index.
[29] Although among the earliest created for the purpose of conformational analysis, the Barton modelsm proved overall much less popular than the Dreiding models, introduced in the late 1950s (Dreiding 1959). A cheaper plastic and metal version of the Dreiding design was introduced by Louis Fieser, for educational purposes, a few years later (Fieser 1963).
[30] Barton took the data for bond length from Linus Pauling’s book, The Nature of the Chemical Bond, considered at the time one of the most authoritative books on matters of molecular structure.
[31] Methods to obtain such values by vector analysis were introduced as early as 1955 (Corey and Sneen 1955), yet it seems that some chemists preferred to stick to the modelm method.
[32] A similar method was described by Langridge et al. (1960) to extract atomic coordinates from modelsm of postulated DNA structures. These coordinates were then used to calculate Fourier transforms which could be compared to X-ray diffraction data.
[33] For a discussion of the use of molecular modelsm in research on the structure of the polypeptide chain, see Francoeur 1998, chapt. 4.
[34] Which is not to say that it could not have been that way.
[35] A particularly familiar case is the work of Watson and Crick on the structure of DNA. Commenting decades later on the initial publication of their DNA modelc, Crick stated that "the structure is produced like a rabbit out of a hat, with no indication as to how we arrived at it" (1974, p. 767). Watson’s subsequent informal account of their work (1968) reveals the importance of modelsm in the articulation of their modelc of DNA.
[36] While Latour sees in this chain the conditions of possibility for "referential truth" (Latour 1987), I am more inclined, with Lynch, to view such "truth" as a "contingent, certified assessment" of a sequence of overlapping elements and practices ((Lynch 1998)). For a study of how the choice of specific structural parameters is made (and criticized) in the design of molecular models, see Francoeur 1998, chapts. 5 & 6.
ApSimon, J.W.; Demarco, P.V.; Raffler, A.: 1966, ‘A Device for the Measurement of Angles and Distances in Molecular Models’, Chemistry and Industry, 1792-93.
ApSimon, J. W.; Graig, W.G.; Demayo, A.; Raffler, A.A.: 1968, ‘The Measurement of Angles and Distances in Molecular Models. III. A General Device’, Canadian Journal of Chemistry, 46, 809-10.
Baeyer, A. v.: 1885, ‘Ueber Polyacetylenverbindungen’, Berichte der Deutschen Chemischen Gesellschaft, 18, 2269-81.
Barton, D.H.R.: 1948, ‘Interaction Between Non-bonded Atoms, and the Structure of cis-Decalin’, Journal of the Chemical Society, 340-42.
Barton, D.H.R.: 1950, ‘The Conformation of the Steroid Nucleus’, Experientia, 6, 316-20.
Barton, D.H.R.: 1956, ‘Molecular Models for Conformationnal Analysis’, Chemistry and Industry, 1136-37.
Barton, D.H.R.: 1987, ‘How to Win a Nobel Prize: An Account of the Early History of Conformational Analysis’, in: Stereochemistry of Organic and Bioorganic Transformations, ed. by W. Bartmann and K.B. Sharpless, VCH, Weinheim.
Barton, D.H.R.; McCapra, F.; May, P. J.; Thudium, F.: 1960, ‘Long-range Effects in Alicyclic Systems’, Journal of the Chemical Society, 1297-1303.
Borman, S.; Dagani, R.; Rawls, R.L.; Zurer, P.S.: 1998, ‘Chemistry Crystallizes into Modern Science’, Chemical & Engineering News, January 12, 1998, 39-75.
Braude, E. A.; Sondheimer, F.: 1955, ‘Studies in Light Absorption, Part XI’, Journal of the Chemical Society, 3754.
Corey, E.J.; Sneen, R.A.: 1955, ‘Calculation of Molecular Geometry by Vector Analysis. Application to Six-membered Alycyclic Rings’, Journal of the American Chemical Society, 77, 2505-9.
Coxon, B.; Hall L.D.: 1964, ‘The Conformation of Cyclic Compounds in Solution-II’, Tetrahedron, 64, 1685-94.
Crick, F.H.D.: 1974, ‘The Double Helix: A Personal View’, Nature, 248 (451), 766-9.
Cross, A.D.: 1964, ‘Steroids. CCLXII. Spectra and Stereochemistry. XVI. A Study of the Mechanism of Long-Range 19-Proton-6B-Fluorine Coupling in 6B-Fluorosteroids’, Journal of the American Chemical Society, 86, 4011-16.
Derx, H.G.: 1922, ‘Contribution à la Connaissance de la Configuration des Systèmes Annulaires dans l’Espace’, Recueil des Travaux Chimiques des Pays-Bas, 41, 312-42.
Dreiding, A.S.: 1959, ‘Einfache Molekularmodelle’, Helvetica Chimica Acta, 42, 1339.
Eliel, E.L.: 1975, ‘Conformational Analysis: The Last 25 Years’, Journal of Chemical Education, 52 (12), 762-7.
Ferguson, E.S.: 1992, Engineering and the Mind’s Eye, MIT Press, Cambridge.
Fieser, L.F.: 1963, Chemistry in Three Dimensions, Cambridge, MA.
Francoeur, E.: 1997, ‘The Forgotten Tool: The Design and Use of Molecular Models’, Social Studies of Science, 27 (1), 7-40.
Francoeur, E.: 1998, The Forgotten Tool: A Socio-Historical Analysis of the Development and Use of Mechanical Molecular Models in Chemistry and Allied Disciplines, Ph.D. Thesis, Sociology, McGill University, Montreal.
Freifelder, M.: 1965, ‘Molecular Models: A Means of Studying Catalytic Hydrogenations’, Platinum Metal Reviews, 9 (2), 38-42.
Galison, P.: 1997, Image and Logic: A Material Culture of Microphysics, Univ. of Chicago Pr., Chicago.
Hall, L.D: 1964, ‘The Conformation of Cyclic Compounds in Solution, I. Shikimic Acid’, Journal of Organic Chemistry, 62, 64-66.
Hazlehurst, T.H. Jr.; Neville, H.A.: 1935, ‘New Models of Old Molecules’, Journal of Chemical Education, 12, 128-32.
Hoffmann, R.; Laszlo, P.: 1989, ‘Representation in Chemistry’, Diogenes, no. 147, 23-51.
Klein, U.: 1997, ‘Nineteenth-Century Chemistry: Its Experiments, Paper-Tools, and Epistemological Characteristics’, Preprint Vol. 56, Max-Planck-Institute für Wissenschaftgeschichte, Berlin.
Klein, U.: 1999, ‘Techniques of modelling and paper-tools in classical chemistry’, in: Models as Mediators, ed. by M.S. Morgan and M.Morrison, Cambridge UP, Cambridge, pp. 146-67.
Langridge, R.; Marvin, D.A.; Seeds, W.E.; Wilson, H.R.; Hooper, C.W.; Wilkins, M.H.F.; Hamilton, L.D.: 1960, ‘The Molecular Configuration of Deoxyribonucleic Acid II. Molecular Models and their Fourier Transforms’, Journal of Molecular Biology, 2, 38-64.
Laszlo, P.: 1993, La parole des choses ou le langage de la chimie, Hermann, Paris.
Laszlo, P.: 1998, ‘Chemical Analysis as Dematerialization’, HYLE – International Journal for Philosophy of Chemistry, 4, 29-38.
Latour, B.: 1986, ‘Visualization and Cognition: Thinking with Eyes and Hands’, Knowledge and Society, 6, 7.
Latour, B.: 1987, Science in Action, Harvard Univ., Cambridge, Mass.
Latour, B.: 1990, ‘Drawing Things Together’, in: Representation in Scientific Practice, ed. by M. Lynch and S. Woolgar, MIT Press, Cambridge, Mass., pp. 19-68.
Latour, B.: 1993a, La clef de Berlin et autres leçons d’un amateur de sciences, La Découverte, Paris.
Latour, B.: 1993b, ‘Le "pédofil" de Boa Vista – montage photo-philosophique’, in: La Clef de Berlin. La Découverte, Paris, pp. 171-225.
Le Fevre, R.J.W.; Sundaram, A.: 1962, ‘Molecular Polarisability: The Conformations of Ten Cyclic Dibasic Acid Anhydrides Indicated by their Dipole Moments, Molar Kerr Constants, etc.’, Journal of the Chemical Society, 4009.
Lemieux, R.U.: 1961, ‘The Configuration and Conformation of Thymidine’, Canadian Journal of Chemistry, 39, 116-20.
Lenoir, T.: 1998, ‘Inscriptions Practices and the Materialitiy of Communication’, in: Inscribing Science: Scientific Texts and the Materiality of Communication, ed. by T. Lenoir, Stanford UP, Stanford.
Lenz, R.W.; Heeschen, J.P.: 1961, ‘The Application of Nuclear Magnetic Resonance to Structural Studies of Carbohydrates in Aqueous Solution’, Journal of Polymer Science, 51, 247-61.
Luisi, P.-L.; Thomas, R.M.: 1990, ‘The Pictographic Molecular Paradigm: Pictorial Communication in the Chemical and Biological Sciences’, Naturwissenschaften, 77, 67-74.
Lynch, M.: 1991, ‘Science in the Age of Mechanical Reproduction’, Biology and Philosophy, 6, 205-226.
Lynch, M.: 1998, ‘The Production of Scientific Images: Vision and Re-Vision in the History, Philosophy, and Sociology of Science’, Communication and Cognition, 31 (2/3), 213-28.
Marvel, C.S.; Glass, D.: 1938, ‘The Possible Assymetry of a Monosubstituted Cyclononane’, Journal of the American Chemical Society, 60, 1051-53.
McEachern, D.M.; Lehmann, P.A.: 1970, ‘Calculating Dipole Moments and Atom Coordinates Using Molecular Models’, Journal of Chemical Education, 47 (5), 389-90.
Mohr, E.: 1918, ‘Die Baeyersche Spannungstheorie und die Struktur des Diamanten’, Journal für praktische Chemie, 98 (2), 315-53.
Mohr, E.: 1922, ‘Zur Theorie der cis-trans-Isomerie des Dekahydro-naphtalins’, Berichte der deuschen chemischen Gesellschaft, 55, 230-31.
Nace, H.R.; Turner, R.B.: 1953, ‘Dipole Moments and Conformations of Androstane-3,17-dione and of Etiocholane-3,17-dione’, Journal of the American Chemical Society, 55, 4063-66.
Nace, H.R.; Turner, R.B.: 1960, ‘The Dipole Moments of Androstan-17-one, Testan-11-one, and Testane-11,17-dione’, Journal of Organic Chemistry, 25, 1403-4.
Perlin, A.S.: 1964, ‘D-Mannofuranose 2,3-Carbonate. Preferred Size of the Sugar Ring in Some Fused-Ring Derivatives’, Canadian Journal of Chemistry, 42, 1365-72.
Ramsay, O.B.: 1974a, ‘Molecules in Three Dimensions (I)’, Chemistry, 47 (1), 6-9.
Ramsay, O.B.: 1974b. ‘Molecules in Three-Dimensions (II)’, Chemistry, 47 (2), 6-11.
Ramsay, O.B.: 1975, ‘Molecular Models in the Early Development of Stereochemistry’, in: Van’t Hoff-Le Bel Centennial, ed. by O.B. Ramsay, American Chemical Society, Washington, DC, pp. 74-96.
Ramsay, O.B.: 1981, Stereochemistry, Heyden, London.
Rheinberger, H.-J.: 1994, ‘Representation(s)’, Studies in the History and Philosophy of Science, 25 (4), 647-54.
Rheinberger, H.-J.: 1997, Toward a History of Epistemic Things, Stanford UP, Stanford.
Richardson, J.S.; et al.: 1992, ‘Looking at Proteins: Representations, Folding, Packing, and Design’, Biophysical Journal, 63, 1186-1209.
Russell, C.A.: 1975, ‘The Origins of Conformational Analysis’, in: Van’t Hoff-Le Bel Centennial, ed. by O.B. Ramsay, American Chemical Society, Washington, DC, pp. 159-78.
Sachse, H.: 1890, ‘Über die geometrischen Isomerien der Hexamethylenderivate’, Berichte der Deutschen Chemischen Gesellschaft, 23, 1363.
Sachse, H.: 1892, ‘Über die Konfigurationen der Polymethylenringe’, Zeitschrift für physikalische Chemie, 10, 203-41.
Smith, D.K.: 1960, Bibliography on Molecular and Crystal Structure Model. Vol. 14, National Bureau of Standards Monograph, National Bureau of Standards, Washington, D.C.
Walton, A.: 1978. Molecular and Crystal Structure Models, Ellis Horwood, Chichester, Hants.
Watson, J.: 1968, The Double Helix, Athaneum, New York.
Wightman, W.A.: 1925, ‘The Spatial Structure of cycloParaffins. Part I. A New Aspect of Mohr’s Theory and the Isomerism of Decahydronaphtalene’, Journal of the Chemical Society, 127, 1421-25.