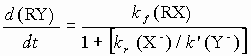
Keywords: fuzzy logic, fuzzy model, possibilistic reasoning, physical organic chemistry, reaction mechanism.
Classification along fuzzy lines as opposed to crisp lines possesses both advantages and disadvantages: in this account we will focus on the advantages. For example, is it meaningful to suggest that there is some different essence in the class of amphoteres that makes them sharply distinctive from both the acids and the bases? Under forcing conditions even the strong base OH- can donate a proton to another base thus exhibiting amphoteric character.
Process control devices utilizing fuzzy algorithms have been used for some time in chemical/biochemical engineering. Industrial and academic research chemists will now encounter in their laboratories both hardware programmed with fuzzy algorithms and software functioning via fuzzy rules.
Classification of input sensor data into small groups of overlapping data sets rather than into a larger number of non-overlapping sets is useful in programming real world controlling devices. Many scientists are unconvinced, however, by the success of fuzzy logic. They feel that it is simply a trendy shorthand redescribing of a fundamentally deeper principle and that it owes its practical success simply to the inadequacy of current measuring devices and the inadequacy of current computing devices. Given better algorithms and more computer power, surely, they think, control devices based on classical crisp logic will come into their own. The leader in the field Lotfi Zadeh (1981) has always insisted that, despite the similarity, there is a precise mathematical difference between the concepts of probability and possibility. He is supported by Ross (1995).
In conventional scientific theories or conventional control systems crisp data is fed into a system and processed via equations: algebraic, difference or differential. The advantage of this system for scientific research is the possibility that the mathematics may throw up some totally unexpected conclusions or actions: the model may predict the existence of black holes in the universe or that there is an ‘island of stability’ in the Periodic Table for elements of atomic number approximately 126. The disadvantage for controlling devices is that the imprecise nature of real data may amplify errors, and unexpected or erratic actions may result from the processing of the calculations. The first approach of fuzzy set theory was from the viewpoint of artificial intelligence, deriving inferences from vaguely formulated data. In the first generation (Linguistic Model, LM type) of fuzzy logic controllers, the input data is fuzzified by grouping it into manageable overlapping sets, then it is processed by inspection of a series of logical but linguistically framed rules of the type:
Many real world LM controllers gave better results than classical logic controllers did 1975-1985. I will argue that the adoption of fuzzy principles by the Hughes-Ingold program 1933-1940 led to scientific advance at a time when the classical paradigm, based on classical reasoning, had stalled due to problems of the fuzziness of the data. However, second generation fuzzy logic controllers now exist (TSK or Takagi-Sugeno-Kang type; Yager & Filev 1994) in which the input data variables are classified into fuzzy sets but then the aforementioned ‘pathways’ subsequently chosen involve the use of classical mathematical equations i.e.
| RX ¬¾® R+ + X – then R+ + Z-¾® RZ | (SN1) | |
| Z- + RX ¾® (Z…R…X)- ¾® ZR + X- | (SN2) |
It is not merely with the benefit of hindsight that we can recognize the crisp/fuzzy dichotomy compared with modern multi-faceted classification (see for example Jones 1984). A rival, crisper classification of organic nucleophilic substitution reactions was proposed in 1939 by Saul Winstein with a five mechanism classification one year before the Hughes-Ingold theory became the center of attention as a result of their powerful consecutive series of papers (Hughes & Ingold 1940; for further technical details of Hughes-Ingold model and Winstein’s model, see Akeroyd 2000).
In principle the transition state for a bimolecular collision can be reduced to a system of equations (see Lewis, McLaughlin & Douglas 1985 on the Marcus Equation, provided that steric effects are discounted), so the spread of products is determined intrinsically from the energy levels of the components of the transition state, a problem that can be solved mathematically by the researcher, provided s/he has correct data to input and access to powerful computers. In contrast, the predictions of the original Hughes-Ingold model followed a few simple rules analogous to the linguistic statements of a First Generation Fuzzy Controller:
When three different alkyl groups are substituted at carbon atom 1 of an alkyl halide the molecule exists in two distinct isomers which are not superimposable on each other, the d and l forms. These forms exhibit optical activity; i.e. they differently rotate the plane of polarized light. Secondary alkyl halides of the type R1R2HCX also exhibit optical activity and on hydrolysis sometimes yield alcohols with complete inversion of configuration, (i.e. complete d > l or l > d conversion), sometimes partial racemization with inversion (incomplete conversion), sometimes full racemization (approximately 50:50 mixture of d and l).[2]
While traditional bimolecular transition state theory possessed a conceptual problem when explaining the frequent observation of full racemization, Hughes and Ingold had a conceptual problem when explaining the not infrequent phenomenon of partial racemization (Akeroyd 2000). This was ‘solved’ by the proposal that for a relatively unstable carbocation R+ with a short ‘lifetime’ before reaction with a water molecule, the departing halide ion would exert a ‘shielding effect’, hindering attack of solvent molecules from that direction.
From 1937 onwards, leading American physical organic chemists abandoned singularity of mechanism but continued opposition to the Hughes-Ingold scheme. In particular they mocked the concepts of ‘carbonium ion lifetime’ and ‘shielding effect’ of the leaving group as ‘vague’ and ‘difficult to quantify’ (Doering & Zeiss 1953, Gould 1959b, Swain et al. 1951, 1955a, 1955b). In our day, they could well have described them as fuzzy concepts containing linguistically imprecise terms.
They also might have objected philosophically that Hughes and Ingold were including extrinsic factors into the explanation of the mechanism of a chemical reaction: the movements of a particular ‘shielding ion’ will be totally arbitrary, determined by solvent effects and presumably obey statistical laws independent of the energy levels of the newly formed carbocation. They therefore pursued the logic of the transition state theory, while conceding the force of experimental results and some of the power of a visualizable model. In addition, they dismissed on energetical grounds the concept of a ‘free’ carbocation, and argued there must be effects from solvent molecules. Hughes and Ingold also agreed that the carbocations must be stabilized by solvent molecules, but they regarded this stabilization as a bulk aggregate effect, similar to the stabilization of alkali metal ions, and that there was no point in including ‘nearby’ solvent molecules in any stage of the mechanism.
Hammett and his PhD student Steigman (1937) proposed two types of polymolecularreaction mechanism for solvolyses:
Two years later Winstein (1939) put forward his own theory, rejecting the concept of SN1 ionizations and the ‘shielding effect’ as an explanation for racemization problems. He proposed a spectrum ranging from mechanism I (identical with Hughes-Ingold SN2) for substitution by a negative ion, through a combined backside/frontside solvated transition state with the chlorine atom eventually being pulled off by the hydrogen bonding of a third alcoholic solvent molecule (II) and finally to a ‘frontside’ bimolecular transition state V which allowed retention of configuration.
We can admire the initial conception of the Winstein mechanistic spectrum. However, in this period there were none of the modern devices capable of detecting the presence of transient species, conformations of transition states, radioactive ‘label’ atoms. Physical organic chemistry relied on kinetic studies, studying the rate of the reaction under investigation and the spread of resulting products. Given the problems of fuzzy data and limited techniques, it is arguable that an appropriately constructed model to cope with the fuzzy data would be more effective than an over precise model, delivering only apparent precision while in fact amplifying errors.
I suggest that Hughes and Ingold accepted in principle the Fuzzy Rules 1, 2, 3, and 5 listed Ross (1995) when generating a heuristic for their ‘research program’. In paraphrased form they run:
This seems straightforward enough but one of the problems is that when the nucleophile is present in large excess the above second order relationship simplifies into that of the first order equation: the reaction is then said to exhibit ‘pseudo first order kinetics’.[3] This could be the case when tert-butyl bromide is hydrolyzed and also in many other reactions at the concentrations used by Hughes and Ingold 1933-1937.
However, after 1937, following a procedure initially published by the
US physical organic chemists Hammett and Roberts (1937), they redescribed
the kinetics for the mechanism for a SN1
hydrolysis in the form
|
|
|
||||
| RX |
¾® |
R+ + X- |
|
RY | |
|
|
– X- |
with the rate
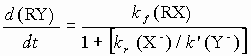
When, however, the concentration of X- is not insignificant and kr ~ k’ then deviations from first order kinetics might be expected (the ‘common-ion’ or ‘mass law’ effect). If the mechanism of hydrolysis was SN2 via attack on RX by neutral water molecules then the reaction
If a reaction is following first order kinetics, or second order kinetics with the second reagent in large excess, the graph ln (A/A0) against t is a straight line (Fig. 1; A is the current concentration of RX, A0 is the original concentration of RX, t is the time).[4]
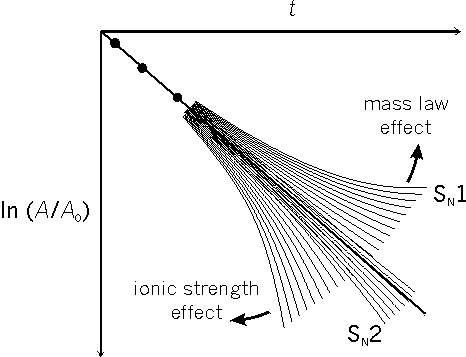
For a reaction obeying first order or pseudo-first order kinetics in the absence of both ionic strength effects and mass law effects it is possible to draw a straight line and make crisp predictions that the rest of the future plots will fall within a probabilistic boundary range. If ionic strength is steadily increased during the reaction, for example by formation of the ions H+ and X-, then it is possible to estimate (using Debye-Hückel Theory) the widening curved probable range.
However, although it is possible to devise a precise mass law equation to represent the mass law effect, there are too many rate constants included in it which may vary in different directions as the ionic strength changes in relation to the developing amounts of H+ and X-. It is impossible to predict, on the basis of initial plots, the range within which future plotted points must fall for the experiment to be considered successful. The most that one can reasonably expect is that the points will fall consistently on at least one of a family of possible curves.
In the case of reactions following the SN2 mechanistic pathway, there is a restriction on the number of possible curves (increasing ionic strength causing a small increase in the velocity of reaction), whereas if the mechanism is SN1 there are a greater number of possible curves depending on whether the mass law effect is greater or lesser than the ionic strength effect. Any plot, drifting to the right in the resemblance of a curve, is fuzzy but important evidence for a preliminary bond breaking step in the mechanism. For a period of some twelve years, there was evidence for the simple and fuzzy Hughes-Ingold concept of all non SN2 reactions operating via a ‘free’ carbocation R+ with a conveniently ‘short’ or ‘reasonably short’ or ‘long’ ‘lifetime’. Before 1940, American physical organic chemists objected to the concept of an ionic mechanism in solvolytic reactions; from 1940 onwards, they objected to a fuzzy interpretation of an ionic hypothesis. While admitting the empirical kinetic evidence for a preliminary bond breaking step in the nucleophilic substitution reactions of the tertiary aliphatic compounds, they refused to accept the unmodified theory in its simple form.
Winstein started to work on the ‘Intimate Ion Pairs’ and ‘Solvent Separated
Ion Pairs’ hypothesis for SN1 reactions.
This led to the American ‘modified Hughes-Ingold system’ overtaking the
basic unaltered Hughes-Ingold system as continued by its progenitors.
In general, it can be said that unwarranted structural assumptions imposed on the working model can lead to dangerous artefacts that do not reflect anything that is contained in the real world data: this leads consequently to meaningless results […] we may still be able to provide answers to our questions such as: what structural relationships between the individual items analysed must exist? Which ones may exist? Which cannot exist? Which may exist perhaps if […]? These modal terms in which we all think, but which we usually rule out of our ‘scientific discourse’, are in fact the proper terms for possibilistic systems. [Bandler & Kohout 1981, pp. 219-220]
A. ‘One or more distinctive heterolytic pathway mechanisms sometimes operating in organic reactions.’
Q. ‘What relationships in the analyzed data may exist?’
A. ‘A simple mechanistic pathway involving the concept of ‘free’ carbocations.’
Q. ‘Which relationships in the analyzed data cannot exist?’
A. ‘The relationship that the mechanism of all organic reactions can be described by transition state theory (SN2 mechanism).’
Now this is a pretty big assumption and, although we know that this concentration of R+ can neither steadily increase nor steadily decrease monotonically throughout the reaction,there is no reason why it should not fluctuate systematically between an upper bound and a lower bound in the course of the reaction. Such reactions have been observed and catalogued (‘oscillator’ reactions) where fluctuations are so gross as to lead to widely different product mixes depending on the time when the reaction is stopped. In the case of the Hughes-Ingold solvolytic curves, I am merely suggesting that fluctuations in the concentration of R+ lead to indeterminacy in the system: no two successive experimental runs will correspond with an ideal graph even in theory.
However, physical detection of carbocationic intermediates was beyond the technical apparatus of the time and similar support for the Hughes and Ingold program was bound not to be forthcoming. This example is the only one I am aware of where possibilistic reasoning was used to make leading scientists switch hypotheses, rather than simply concede that ‘such and such a theory has considerable merit’ or ‘such and such a theory is certainly making headway’.
One reason for the success of the early Linguistic Model Controllers
based on Zadeh’s logic was their ability
to incorporate the experience of inarticulate human operatives of the system
in question into a machine programmable form. Earlier classical controllers
based on equations were unable to do this as successfully. In a sense the
simple fuzzy Hughes-Ingold model successfully incorporated the experience
of previous generations of organic chemists, who, as is well-known, had
been proceeding in a highly atheoretical manner from 1860-1930 (see for
example Gould 1959c). One can therefore understand its success and also
the reluctance of Hammett, Roberts, and Winstein to abandon the mathematics
entirely in their model and therefore to try and build up a hybrid system
containing the best of the old and the best of the new: i.e. a system
resembling the Takagi-Sugeno-Kang (TSK) controllers using both fuzzy principles
and classical equations.
[1] Winstein’s (1956) Dual Ion Pair hypothesis is still accepted today (see Lund 1995). It was immediately criticized as being fuzzy by Hughes and Ingold (1957) in a paper received by the Journal of the Chemical Society on Oct. 5, 1956, and Jones (1984), in an undergraduate text, comments "because it is so flexible it leaves room for doubts about the precise mechanisms of reactions that fall between clear-cut extremes." Unlike the Hughes and Ingold model, the Winstein model received experimental corroboration almost immediately via the tool of isotopically labelled oxygen atoms (Denney 1957).The analogy with the TSK controllers can be seen by inspecting the equations given on p. 329 of Winstein (1956), which can be reformulated using the IF … THEN rules of TSK, i.e
IF ksIII >> k-2[2] Hydrolysis with retention of configuration is possible if one of the R groups contains another substituted heteroatom Y. In this account, we are discussing normal CnH2n+1 type R groups.
THEN equation (6) degenerates to kt = k1[ 1 + k1/k2]
ELSE …[3] Other problems arise if the total concentration of the nucleophile is made up of associated ion pairs and ‘free’ ions e.g. Na+OH-« Na+ + OH-.
At higher concentrations, estimating the amount of total base remaining will not reflect the concentration of the active species OH- that is responsible for affecting the rate of the reaction. Departures from second order kinetics are expected which may approximate to first order kinetics.
[4] For convenience Hughes & Ingold often worked with the expression k1 = (2.3/t) log10 (A0/A) rather than the ‘classical’ plots used here (see Journal of the Chemical Society, (1933), p. 1573).
Akeroyd, F.M.: 2000, ‘The rise of the Hughes & Ingold Theory from 1930-42’, Foundations of Chemistry, 2, 99-125.Bandler, W.; Kohout., L.J.: 1981, ‘Semantics of implication operators and fuzzy relational products’, in: E.H. Mamdani & B. R. Gaines (eds.), Fuzzy Reasoning and its Applications, Academic Press, London, pp. 219-46.
Bartlett, P.D.; Knox, L.H.: 1939, ‘Bicyclic Structures Prohibiting the Walden Inversion’, Journal of the American Chemical Society, 51, 3184-92.
Chang, R.: 1998, Chemistry, 6th edn., Mc Graw-Hill, New York, pp. 317, 628.
Denney, D.B.; Goldstein, B.: 1957, ‘An Oxygen-18 Tracer Study of the Rearrangement in 2-Pheny-1-Propyl Bromobenzenesulphonate’, Journal of the American Chemical Society, 79, 4948-51.
Doering, W.; Zeiss, H.H.: 1953, ‘Methanolysis of Optically Active Hydrogen 2,4 dimethylhexyl-4-pthalate’, Journal of the American Chemical Society, 75, 4733.
Gaines, B.R.: 1976, ‘Foundations of Fuzzy Reasoning’, International Journal of Man-Machine Studies, 6, 623-68.
Gould, E.S.: 1959a-c, Mechanism and Structure in Organic Chemistry, Holt, Rinehardt and Winston, New York, (a) pp. 299-302, (b) p. 269, (c) p. 2.
Hammett, L.P.; Roberts, I.: 1937, ‘The Mechanism of Substitution Reactions: Benzyl Chloride with Mercuric Salts’, Journal of the American Chemical Society, 59, 1063-8.
Hughes, E.D.; Ingold, C.K.: 1940, ‘Mechanism of substitution at a Saturated Carbon Atom. Parts XI-XXIII, Journal of the Chemical Society, 913-1024.
Hughes, E.D.; Ingold, C.K.; Mok, S.F.; Patai, S.; Pocker, Y.: 1957, ‘Mechanism of substitution at a Saturated Carbon Atom. LVIII.’, Journal of the Chemical Society, 1265-70.
Jencks, W.P.: 1981, ‘How does a Reaction choose its Mechanism?’, Chemical Society Reviews, 10, 345-65.
Jones, R.A.Y.: 1984, Physical and Mechanistic Organic Chemistry, 2nd edn., Cambridge University Press, Cambridge, pp. 149-150.
Lewis, E.S.; McLaughlin, M.L.; Douglas, T.A.: 1985, ‘Methyl tranfers. 10. The Marcus Equation. Application to Soft Nucleophiles’, Journal of the American Chemical Society, 107, 6668-73.
lund, H.; Daasbjerg, K.; Lund, T.; Pedersen, S.U.: 1995, ‘On Electron Transfer in Aliphatic Nucleophilic Substitution’, Accounts of Chemical Research, 28, 313-9.
Ross, T.J.: 1995, Fuzzy Logic with Engineering Applications, McGraw-Hill, New York, pp. 474-5.
Steigman, J.; Hammett, L.P.: 1937, ‘Solvolytic Reactions of alpha-Phenylethyl Chloride’, Journal of the American Chemical Society, 59, 2536-42.
Swain, C.G.; Langsdorf, W.P.: 1951, ‘Concerted Displacement Reactions. VI’, Journal of the American Chemical Society, 73, 2813-9.
Swain, C.G.; Kreevoy, M.M.: 1955a, ‘Mechanism of Anion Exchange in Trityl Chloride in Benzene’, Journal of the American Chemical Society, 77, 1122-8.
Swain, C.G.; Moseley, R.B.: 1955b, ‘Rates of Solvolysis of Tri phenyl Methyl Fluoride’, Journal of the American Chemical Society, 77, 3727-31.
Winstein, S.: 1939, ‘the Solvolysis of t-butyl chloride’, Journal of the American Chemical Society, 61, 1635-9.
Winstein, S.: 1942, ‘The Role of Neighbouring Groups in Replacement Reactions. I-V’, Journal of the American Chemical Society, 64, 2780-1 (2780).
Winstein, S.: 1956, ‘Salt Effects and Ion Pairs in Solvolysis and Related Reactions’, Journal of the American Chemical Society, 78, 328-35.
Yager, R.R.; Filev, D.: Fuzzy Modelling and Control, J. Wiley, New York, 1994, pp. 192-4.
Zadeh, L.A.: 1981, ‘PRUF – a meaning representation language for natural languages’, in: E.H. Mamdani & B.R. Gaines (eds.), Fuzzy Reasoning and its Applications, Academic Press, London, pp. 1-66.
F. Michael Akeroyd:
Bradford College, BD7 1AY, U.K.; m.akeroyd@bilk.ac.uk
Copyright Ó 2000 by HYLE and F. Michael Akeroyd