‘Exalting Understanding without Depressing Imagination’
Depicting Chemical Process
David Knight*
Abstract: Alchemists’ illustrations indicated through
symbols the processes being attempted; but with Lavoisier’s Elements (1789),
the place of imagination and symbolic language in chemistry was much reduced.
He sought to make chemistry akin to algebra and its illustrations merely
careful depictions of apparatus. Although younger contemporaries sought,
and found in electrochemistry, a dynamical approach based upon forces rather
than weights, they found this very difficult to picture. Nevertheless,
by looking at chemical illustrations in the eighty years after Lavoisier’s
revolutionary book, we can learn about how reactions were carried out,
and interpreted, and see that there was scope for aesthetic judgement and
imagination.
Keywords: visualization of chemical process, chemical
manipulation, laboratory apparatus, textbook illustrations.
1. Chemistry as poetry, realized
Friedrich Schlegel used the method or logic of chemistry, illuminating
mixture and combination, in his romantic fragments (Chaouli 2002, pp. 27-9),
thus taking the science into literature. Samuel Taylor Coleridge declared[1]
that in the work of the chemists Humphry Davy, Charles Hatchett, and William
Hyde Wollaston "we find poetry, as it were, substantiated and realized
in nature: yea, nature itself disclosed to us […] as at once the poet and
the poem". The key was imagination; and he quoted Shakespeare[2]
Lovers and madmen have such seething brains,
Such shaping fantasies, that apprehend
More than cool reason ever comprehends.
The lunatic, the lover, and the poet,
Are of imagination all compact.
The chemist in dynamical relationship with matter looked very different
from the chemist in the popular image of today, when ‘chemical’ is set
against ‘natural’, or ‘organic’, and associated (despite all the poisonous
animals, vegetables, and minerals in the world) with danger to ordinary
people, artificiality, forbidden knowledge, and weapons. Imagination and
enthusiasm were conveyed about 1800 in demonstration lectures, which might
even have a slight spice of danger from explosions or liberated gases.
However, it seems worth investigating how, in the eighty years before about
1870, such chemical dynamics could be got across on the printed page, and
what was then its aesthetic impact. Looking at illustrations may anyway
cast light on what chemistry was like in these years, before its dynamics
could be much understood. It may help us in various degrees to appreciate
what was understood about the chemical process, how chemistry was perceived
as an inductive science, and to see the chemist at work in the experiments
which were so central to the science.
Physicists have long opposed dynamics, the science of forces, to statics;
and also (since the mid-nineteenth century in English) to kinematics (Little
et
al. 1964, vol. 2, pp. 575, 1086), as the science of pure motion, considered
without reference to masses or forces. One can thus say that Tycho’s analysis
of planetary motions was kinematically the same as Copernicus’, with the
signs changed, while dynamically it was very different, with its requirement
that the Sun carrying all the planets moved in orbit round the Earth. In
chemistry, on the other hand, and generally in discussions in the Romantic
period, dynamical science was opposed to the study of masses and recipes,
and went along with interest in Natura naturans rather than Natura
naturata.
2. A dynamical chemistry
Davy, when he wrote at the end of his life that "whilst chemical pursuits
exalt the understanding, they do not depress the imagination or weaken
genuine feeling" (Davy 1830, p. 245), was recycling material from his lectures
at the Royal Institution into a kind of testament or legacy, which was
posthumously published as Consolations in Travel. With its beginning
in the Colosseum, the book recalls the ancient world and Boethius, whose
Consolations
of Philosophy, written by another eminent man facing death, had been
first translated into English by King Alfred. Davy had sought to show,
in performance at the Royal Institution (Knight 2002) and in his writing,
that chemistry was not simply an ‘art’, a series of useful techniques to
be mastered like advanced cookery, but involved the highest faculties,
and was indeed creative. The chemist was neither a mere artisan nor simply
an analyst, an accountant of matter, seeing what things were made of and
balancing his equations; he was godlike, manipulating the powers of nature,
making new substances, improving the world (Knight 2003).
He might also be manly, even macho (Knight 2000), a master interrogating
nature with his instruments, rather than a passive scholar. He had to think
with his fingers. Chemistry required manual skills in ways that astronomy
or mathematical physics did not, and was a science of secondary qualities
– colors, tastes, textures, and smells, hard to describe exactly, but once
experienced, unforgettable. It was beautiful, and also dynamically unstable,
and labile. Affinities were revealed and expressed through the chemical
process: few substances were incorruptible, like gold, and everywhere chemical
change was going on with the inexorable passage of time.
Davy learned his chemistry in the 1790s (Fullmer 2000), through Thomas
Beddoes in Bristol whom he assisted at the Pneumatic Institution, set up
with Josiah Wedgwood’s money and James Watt’s expertise to see whether
Joseph Priestley’s factitious airs might be medically useful. These three
had all been members of the Lunar Society of Birmingham (Uglow 2002), in
eclipse after Priestley’s house was sacked by a ‘Church and King’ riot
in 1791. They shared with their protégé Beddoes a sympathy
with the French revolutionaries which marked them out as dangerous subversives
in the climate of world war. In 1794 Priestley had been forced into exile
in the USA, while a police spy trailed Beddoes’ friends, the poets Coleridge
and William Wordsworth.
Men of science in France, philosophes, were perceived in Britain
to have been responsible for the revolutionary ideology now threatening
the ‘sceptered isle’. For Sir Joseph Banks, President of the Royal Society,
and his friends it was essential to establish that science was compatible
with the British model of constitutional representative government (Gascoigne
1998). This went with an emphasis (congenial to Britons of the time) upon
utility, and upon cautious Baconian generalization rather than broad-brush
theorizing (Smith 1994). When Davy moved on to London, and his astonishingly
successful career and social mobility, he took pains to distance himself
from democrats and emphasized how the unequal division of property made
economic and scientific progress possible.
However, with his work on electrochemistry leading to his hypothesis
that ‘electrical energy’ and chemical affinity were identical (Davy 1839-40,
vol. 5, pp. 39-40), Davy was also an important pioneer of dynamical science
against the Newtonian clockwork universe, which had been given new life
in William Paley’s Natural Theology of 1802. Chemistry was a science
that appealed to Romantic writers such as Coleridge, who went to Davy’s
lectures to improve his stock of metaphors; and Percy and Mary Shelley
– in Mary’s
Frankenstein (Shelley 1994), Professor Waldman who enthused
young Victor echoed the rhetoric of Davy, whom she knew. Chemistry went
well with the romantic active universe: thus in Germany, not only with
Schlegel (Chaouli 2002, pp. 27-9) and what he believed to be a kind of
chemical logic, but also with Johann Wolfgang Goethe’s Elective Affinities
(Goethe 1971) where the adventures of the humans (falling in and out of
love) parallel the chemistry they are studying. It is not only the visual
arts that have chemical connections; we may still speak of our ‘chemistry’
as responsible for our moods, and use words like ‘catalyst’ metaphorically.
Words do resonate, but the imagination is quickened especially by visual
symbols.
3. The rich language of symbols
In Antoine Lavoisier’s work, the science had turned its back on its speculative,
alchemical past; but chemical philosophers were still intrigued by their
inheritance. Davy was among those who thought that metals might be complex
and transmutable, and even compared his newly discovered potassium with
the alkahest (Davy 1839-40, vol. 5, pp. 66, 89). Alchemists had had a very
rich tradition of illustration (Principe & De Witt 2002, p. 8). In
the Orthodox Churches of the East, theology was expressed in icons, where
truths about God and His dealings with the world could be conveyed visually
when ordinary language fell short and Western thinkers got entangled in
logic (Armstrong 1999, pp. 256-9). Similarly, Nature’s workings (and the
chymists’ efforts to quicken them, ripening metals more speedily) might
be better depicted than described (Vertesi, no year): "A picture is not
merely ‘worth a thousand words’; a picture can tell us more than words
alone can effectively express." The genre paintings of alchemists’ workshops
or laboratories often seem to portray a team at work, though the focus
is upon their leader. They may indicate either futility and bankruptcy,
perhaps with the chymist’s ruined family in evidence, or deep scholarship
and a kind of tranquillity achieved through activity.
Modern chemistry was rather different, but symbolism is there. Jacques
Louis David’s famous and triumphant portrait of Lavoisier and his wife
has been shown to merit close study because of what it manifests: its affinities
to a canonical portrait of Descartes, the curious way in which the sitters
are very formally dressed but apparently in the laboratory with identifiable
apparatus on display, and the look that he is giving to her (perhaps as
his muse) all make it extremely striking (Beretta 2001), indeed stunning,
and have ensured that it has eclipsed other portraits of Lavoisier. Perhaps
from our point of view, however, the plates at the back of his Elements
of Chemistry are more significant (Lavoisier 1790). His idea was to
portray exactly what apparatus he had used, and how, so that anybody could
repeat his experiments and thereby come to his theoretical conclusions:
but the plates were not merely illustrative, but handsome.
4. An exact and sober science of weights
Lavoisier had no need to spare expense, being indecently wealthy.[3]
Indeed to duplicate the equipment in his laboratory would have been impossible
for most people or institutions. In his book, one of his intentions was
to replace the rich, suggestive, and ambiguous language of earlier chemistry
with a precise (almost Linnaean) nomenclature akin to algebra. Metaphor,
echo, different levels of meaning, poetry, or coded messages – imagination
indeed – had in principle no place in this classic work of the Enlightenment.
In the same way, the plates (which are copper-plate engravings) are like
accurate topographical art: they are descriptive rather than interpretative,
even though they form part of a book specifically designed to bring about
a revolution – Lavoisier being one of the first men of science to use that
word in the modern sense, and of science. Publishing in 1789, he knew that
he was doing in the realm of chemistry what he then hoped and believed
that the reformers were doing in France as the Estates General was convened,
and the Bastille fell. Unfortunately, by the fourth edition of 1799 the
translator could deplore the death of the author on the guillotine at the
hands of the sanguinary monster and tyrant Robespierre (Lavoisier 1799,
p. xi).
The third section of the book, occupying pages 291 to 479, is devoted
to the instruments and operations of chemistry, but it is the former that
lend themselves to illustration in the thirteen plates that follow. There,
the flasks and other apparatus are carefully shaded, so that we get the
impression of perspective and of their shape in three dimensions. For the
larger objects there is a scale of feet, so that we can see how big the
pieces are. It is clear in the plates what is made of glass, and what of
wood. Complicated pieces are shown in cut-away form, so that we can see
the inside. The coiled worm of tubing forming a condenser is shown in dotted
lines within its vessel of cold water, and some long chains of apparatus
are shown linked together. There are pestles and mortars, and files, and
carefully fluted filter-papers.
5. Forces and equilibria
For capturing the chemical process, as alchemists had sought to do with
lions, kings, and serpents, Lavoisier’s plates are not very helpful, though
there is conventional fire beneath some retorts or alembics, a tube or
gun-barrel being kept hot in a furnace, and an illustration of the sun’s
rays being concentrated by a lens. Since a furnace with an oxygen blast
is among the equipment available, there was every chance of some dramatic
experiments. However, unlike several eminent chemists, Lavoisier seems
to have avoided serious personal injury in what Davy perceived as the service
of danger in the laboratory. Although Lavoisier was greatly concerned with
the role of heat in chemical reactions (and professionally at the Arsenal
with explosions), his chemistry of the balance sheet (Holmes 2003) lacked
the dynamical emphases which William Odling (perhaps slightly tongue in
cheek) detected in his opponents. Odling sought to rehabilitate Becher
and Stahl, in a lecture on chemical thermodynamics as "The Revived Theory
of Phlogiston", at the Royal Institution in April 1871 (Odling 1870-2).
Phlogiston for him was an anticipation of chemical energy, and seen in
that light its supporters had the right end of the stick.
Lavoisier had worked on heat and chemical reactions, and his associate
Claude Louis Berthollet, the doyen of French chemists in the Napoleonic
years when he was a prime mover in the Society of Arcueil (Crosland 1967),
had a strong feeling for chemical dynamics. He was one of the team of men
of science who went to Egypt with Bonaparte, and while there he set out
his ideas about chemical affinity, announcing them in Cairo in the seventh
year of the republic (1799). They were duly published in a little book,
translated into English in 1804 (Berthollet 1804), with an American edition
in 1809 (Berthollet 1809). The book is surprisingly free from illustrations
or diagrams, unlike those of some predecessors in the study of affinity
(Duncan 1996, pp. 145-8, 201-24), but it did provide an impetus toward
understanding process with its idea that the masses of the reacting substances
were crucial for the outcome.
Berthollet expanded the little book into a two-volume study (Berthollet
1803), and set off a great deal of discussion and major research on definite
proportions by John Dalton and Joseph-Louis Proust (Brock 1992, pp. 144-5),
which led into the world of chemical statics (weights) rather than dynamics.
But Berthollet did not try to illustrate this book either. In his controversy
with Proust he was generally held to have lost, so that, although Davy
felt in 1806 that his electrochemical work supported Berthollet’s ideas
(Davy 1839-40, vol. 5, p. 41), they did not catch on and a chemistry of
weights prevailed. Chemical equations (when they began gradually to come
in) thus expressed masses rather than forces; and as has been recently
remarked:[4]
the usual chemical equations tell us about the atoms that are involved
and about the compositions of the molecules. However, they tell us nothing
about the reactions. In this sense we are still using 19th
century notation in chemistry. There is need for a notation that would
allow us to see that H + Cl2is the same
reaction as K + CH3I. At first glance,
a chemist would not have anticipated that.
6. An algebra of chemistry
Following Maurice Crosland (1978, pp. 227-81), Marco Beretta (1993) has
described the various symbols (some descending from alchemy) used in the
chemistry of the late eighteenth century, which did include those for operations
and processes, such as distillation and sublimation, as well as for apparatus
and substances. However, these seem to be cases where the symbol is just
a shorthand, or maybe an aide memoire,[5] bearing
no resemblance to what is symbolized, and therefore, while more or less
elegant and convenient, not necessarily of any great aesthetic significance.
Though the symbols in Diderot’s great Encyclopedie are rather beautiful,
they would have involved the chemist in learning something like Chinese
characters. They were also problematic on utilitarian grounds: like email
addresses, they lacked the redundancy helpful in ordinary words, where
mild misspelling is not fatal to understanding.
The famous surgeon James Parkinson in his Chemical Pocket-book
(Parkinson 1801) (Figure 1) illustrated some apparatus on his frontispiece
(curiously, set out as in a theatre, with pillars and stage curtains) with
a selection of the symbols of Lavoisier’s pupils Jean Henri Hassenfratz
and Pierre-Auguste Adet. William Nicholson in his Dictionary of Chemistry
gave a full table of this attempt to express chemistry as algebra (Nicholson
1808, plate 4). But although there were symbols for substances yet to be
discovered, there were by this time none for processes. The algebraic tradition
(by then Boolean) was revived in the 1860s by Benjamin Brodie (Brock 1967)
in his ‘Calculus of Chemical Operations’, but despite its title his system
was concerned with an ‘ideal chemistry’ of imaginary operations rather
than symbolizing actual processes. Brodie did at least use Greek letters,
easier to remember than previous hieroglyphs. However, none of these notations
caught on, any more than Dalton’s circles, which were to his chagrin finally
rejected at the British Association for the Advancement of Science’s Dublin
meeting in 1835, in favor of Jacob Berzelius’ much less suggestive alphabetical
notation. Illustrating the chemical process proved very difficult; to show
how things were to be done was easier.
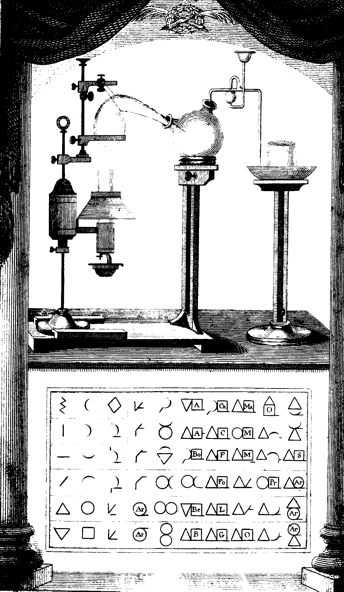
Figure 1: Frontispiece, with apparatus and symbols (from Parkinson
1801).
7. Hands-on chemistry
Davy’s description of his experiment isolating potassium is spirited, but
there is no picture of "the globules [that] often burnt at the moment of
their formation, and sometimes violently exploded and separated into smaller
globules, which flew with great velocity through the air in a state of
vivid combustion, producing a beautiful effect of continued jets of fire"
(Davy 1839-40, vol. 5, p. 62). Nor in his illustrations do we get what
was common in the nineteenth century, the inclusion of disembodied hands
(and occasionally mouths) as an indicator of how apparatus is to be used.
Frequently the plates of apparatus were copied from one book into another,
sometimes getting reversed; and sometimes they were essentially advertisements.
Thus Friedrich Accum’s illustrations include scientific apparatus and equipment
labeled as available from him in 1807, and his book thus has some aspects
of a trade catalogue (Accum 1807, plates III, IV, VI, vol. 2, p. xxiv).
His description of the plates is a useful introduction to chemical manipulation.
While some plates, like that of a test-tube rack, will (though static)
evoke nostalgia among those of us who remember such things still as standard
equipment 150 years after his book was published, some show experiments
going on, and thus have a dynamical aspect. Thus a flame is being blown
by steam in a patent self-acting blowpipe, while in another (advertising
a self-acting thermometer) a bladder with stop-cock is held in a cuffed
hand indicating how to squeeze the air out of it (Figure 2). Hands similarly
appear in the engravings (done in 1809) in Jane Marcet’s Conversations
on Chemistry (Figure 3), where complicated apparatus is also shown
in use (Marcet 1828, plate XI). In Accum’s Chemical Re-agents or Tests,
we actually see both of the chemist’s hands and his mouth (Accum 1828,
plate 3): he is directing a candle flame onto a sample held in a pair of
tongs, using a blowpipe (Figure 4).
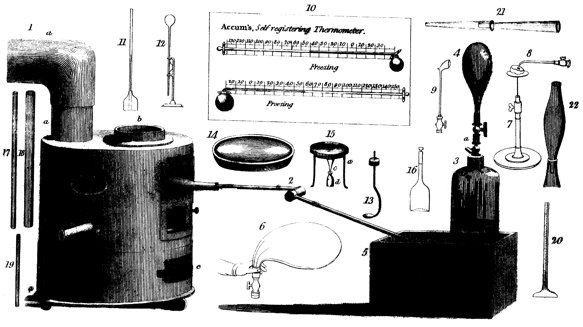
Figure 2: Apparatus including a furnace, a bladder, a clay pipe, and
thermometers (from Accum, 1807, plate VI).
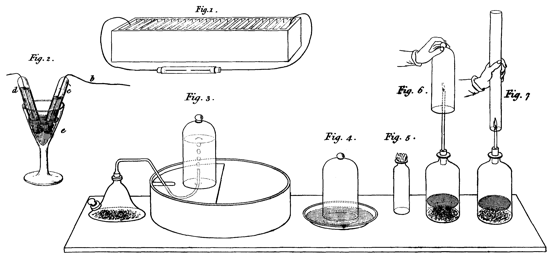
Figure 3: Apparatus for decomposing water and studying hydrogen (from
Marcet 1828, plate XI).
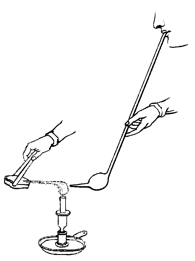
Figure 4: Apparatus (from Accum 1828, plate 3).
Mme. Lavoisier did famous sketches of her husband’s laboratory, where
five people were working on respiration experiments, and she was taking
notes,[6] but active, peopled laboratories are unusual
in art. There are splendid illustrations of laboratories, notably in William
Brande’s Manual of Chemistry (1830) where the frontispiece shows
the Royal Institution’s in the days of Davy and Faraday; and another plate
depicts a portable laboratory, engraved from a drawing by Faraday (Brande
1830). However, neither the fixed nor the portable apparatus is in use.
The laboratory is empty of human interest, as it is in Colin Mackenzie’s
One
Thousand Experiments in Chemistry (Mackenzie 1822) – though that book
has a magnificent colored frontispiece of a gas works with heroic workers
drawing the retorts, and a crescent moon shining through a grated window
overhead, giving a wonderful impression of a process going on (Figure 4a).
Rather weirdly, an alchemical eagle is emerging from an alcohol blowpipe
in his plate 9 (Figure 5). Other illustrations include a jolly little coal
train apparently chugging along without a driver and an elaborate heating
and plumbing system for a house – but there is little feeling of real chemical
dynamics. Faraday’s Chemical Manipulation (Faraday 1842) has excellent
descriptions of doing experiments, but the illustrations (which are woodcuts
scattered through the texts, rather than copperplate engravings), are static,
with the convention as in Lavoisier’s book of showing by dotted lines the
shape of concealed pieces of apparatus.

Figure 4a: Frontispiece, drawing retorts in a gas-works (from Mackenzie
1822).

Figure 5: Apparatus for generating gases, and alcohol blowpipe (Mackenzie
1822, plate IX).
8. Picturing chemical reactions
William Henry’s Epitome of Chemistry (Henry 1803) contains affinity
diagrams in the eighteenth-century manner, but no illustrations. His Elements
of Chemistry, however, which was revised by the popularizer J. Scoffern
as a volume in the ‘Circle of the Sciences’ published about 1852, has fascinating
illustrations of experiments going on (Scoffern 1852, pp. 191, 357). Thus
the effect of putting water into white-hot silver vessels is graphically
depicted (Figure 6). Elsewhere, we find a picture including an experimenter’s
hand, a diagram indicating the play of affinities, and a chemical equation,
between them telling the reader much about the reaction (Figure 7). Faraday’s
friend and admirer J. B. Daniell, whose Chemical Philosophy approaches
the subject in the direction of what we would call physical chemistry,
did something like this too (Daniell 1839, pp. 301). His title emphasized
forces (echoing the ‘causes’ of his colleague Charles Lyell’s Principles
of Geology [Lyell 1830-3]), and rather than atoms (he was a slightly
uneasy admirer of Dalton, fearing that atomists might get lost in metaphysics)
he preferred to think of volumes. His reactions are therefore set out with
little boxes representing volumes of reactants, shown alongside the apparatus,
here for the analysis of ammonia contained in a bladder by blowing it through
a tube containing heated copper (Figure 8). Berzelius, however, in his
work on the blowpipe, kept illustrations and formulae well apart (Berzelius
1845). This seems to have been usual in the middle years of the nineteenth
century, perhaps a high-tide of positivism.
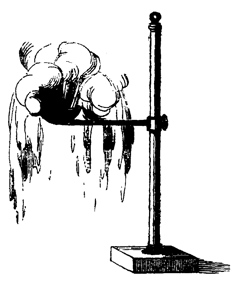
Figure 6: Effects of rapid boiling (from Scoffern 1852, p. 191).
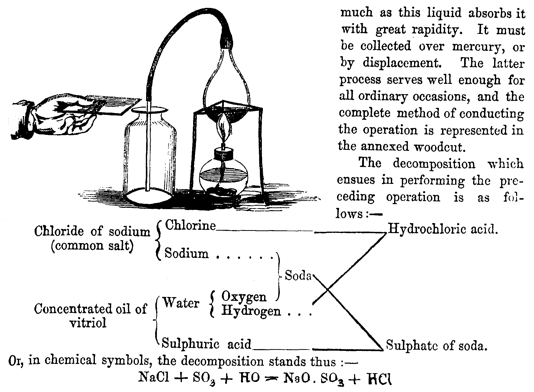
Figure 7: Three ways of depicting the preparation of hydrogen chloride
from common salt (from Scoffern 1852, p. 357).
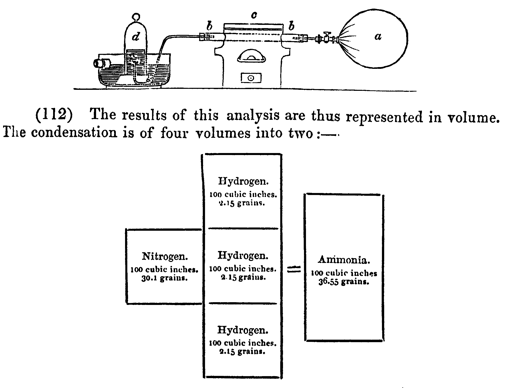
Figure 8: Analysis of ammonia (from Daniell 1839, p. 301).
Alexander Williamson, who had indeed studied with Auguste Comte, in
1851 lectured on his ether synthesis[7] under the promising
title ‘Suggestions for the Dynamics of Chemistry’ and did not make use
of illustrations. But August Wilhelm Hofmann, lecturing in 1862 about the
new synthetic dyes, mauve and magenta (Hofmann 1862), used ‘type moulds’,
wire frames into which little boxes could be put, to bring the ‘type’ theory
to life. In his subsequent lecture on the combining power of atoms,[8]
he showed little tin boxes as "a simple mechanical contrivance" to represent
volumes and demonstrate types and reactions. These were duly illustrated,
rather unexcitingly from the aesthetic point of view, when the lecture
was published (Figure 9). Along with them, there was a very detailed and
carefully-shaded picture of apparatus, showing the grain of the wood of
which the laboratory bench was made, the wooden block upon which the new-fangled
Bunsen burner was raised, the design of the gas taps and the base of the
retort-stand, and the hand of the operator carefully pouring liquid into
the tube (where he would have been wise to have made use of a glass rod)
(Figure 10). But then, with what seems to hindsight an enormous leap forward,
we meet (Figure 11) his
[...] illustration from that most delightful of games, croquet.
Let
the croquet balls represent our atoms, and let us distinguish the
atoms of different elements by different colours. The white balls are hydrogen,
the green ones chlorine atoms; the atoms of fiery oxygen are red, those
of nitrogen, blue; the carbon atoms, lastly, are naturally represented
by black balls.
Into the balls, metal tubes and pins were screwed so that he could build
up models. Soon sets of these ‘glyptic formulae’ were available to arouse
the ire of Brodie as materialistic joiner’s work, unworthy of the attention
of chemists, and set off debates at the Chemical Society of London about
the value and truth of atomic theory (Brock 1967). There was no longer
any doubt that it could generate handsome and heuristically-useful models,
having their own kind of aesthetic appeal, and making chemistry much easier
to learn.
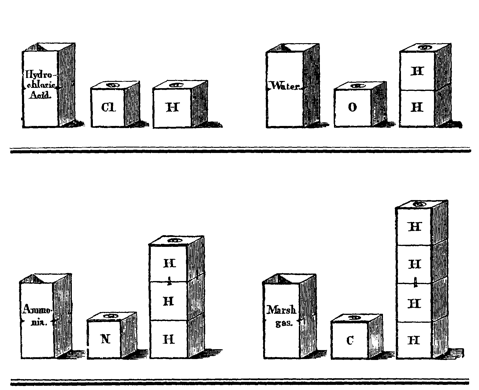
Figure 9: Tin boxes illustrating the type theory (from Hofmann 1862-6,
pp. 412-3).
We have met with some attempts to depict rapid reactions, but the importance
of time in chemical reactions – some explosively fast, others extremely
slow – was only appreciated during the hundred years that followed our
chosen time. Williamson’s insight in his work on ethers that reactions
go in stages could only be developed in the light of the atomic theory
and agreed formulae that were a feature of the 1870s. However, whether
the series of equations, which covered the pages of the journals from the
later nineteenth century and that indicate how syntheses were achieved
and how reactions go, were of aesthetic merit is another question – usually
they are seen as turning-off all but dedicated professionals, and blinding
(rather than enlightening) everyone else with science. Something less austere
is needed to feed the outsider’s imagination.
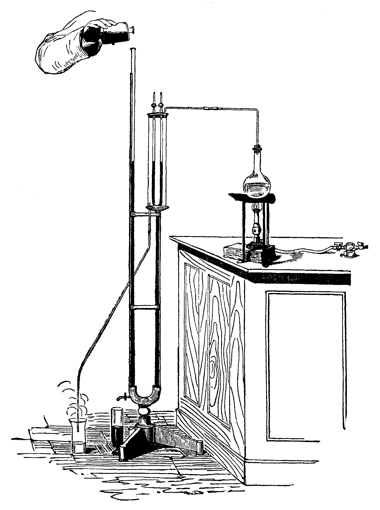
Figure 10: Demonstrating the gas laws (from Hofmann 1862-6, p. 415).
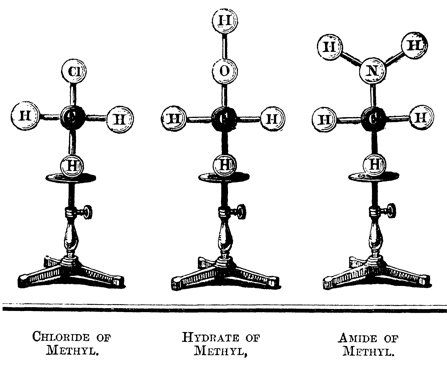
Figure 11: Molecular models (Hofmann 1862-6, p. 426).
9. Finding conceptual tools
We have encountered chemists at work in the laboratory, found visual clues
indicating how experiments were done, and seen some ‘stills’ as it were
from chemical processes like distillation. However, showing movement was
not easy, and our search for depictions of chemical dynamics has not been
very fruitful. To go to lectures and witness experiments was enlightening,
and to listen to the enthusiastic lecturer was exciting. But what was seen
there proved hard to convey in pictures or indeed in vivid prose.[9]
In eighteenth and nineteenth-century works of natural history, natural
historians like Thomas Pennant and J. J. Audubon sometimes sought to portray
a bird not at rest on a branch or twig, but in flight or eating, while
Josef Wolf showed exotic animals fighting.[10] This
was not easy because artists were usually working from stuffed specimens,
with at best a sketch done in the field. Edward Lear’s studies of parrots
were unusual in being done from living specimens, in the newly founded
London Zoo (Jackson 1975, pp. 32-8). As we have seen, it was much more
difficult in chemistry.
As Trevor Levere remarks,[11] "Until the 1870s […]
chemists lacked the conceptual tools to picture and model three-dimensional
molecules". The innovations of Hofmann and then of Jacobus Henricus Van’t
Hoff (Ramberg 2003) allowed interactions to be visualized and depicted
– in a process that has subsequently gone on, most evidently in Roald Hoffmann
and Vivian Torrence’s Chemistry Imagined (Hoffmann & Torrence
1993) with its evocative pictures as in ancient traditions, in association
with structural diagrams, some of considerable elegance and even beauty.
In synthesizing molecules unknown in nature, the kind of creative activity
that Davy talked about, the playful chemist (Nickon & Silversmith 1987)
can prepare ‘churchane’ and ‘barrelene’ – though the beauty of the equations
leading to them is an acquired taste, caviar to the general, and only loosely
connected to ordinary ideas of the aesthetic.
Despite the opposition to atoms and visualization, led notably by Marcellin
Berthelot, Brodie, and Wilhelm Ostwald, Lavoisier’s austere notions of
what was metaphysical and what chemical did not endure beyond the early
twentieth century. Now diagrams are essential, and everyone recognizes
that chemistry is dynamic. To claim that it is poetry realized would surprise
most readers today; but that it requires imagination cannot be doubted.
Notes
[1] Coleridge 1969, vol. 1, p. 471; Levere 1981.
[2] William Shakespeare, A Midsummer-Night’s
Dream, V, I, lines 4-8.
[3] Poirier 1996, pp. 1-3; Donovan 1993, pp.
110-29; and on language, pp. 159-67, but see also Anderson 1984.
[4] Dudley R. Herschbach in Hargittai 2003, p.
396.
[5] The British Museum has in the summer of 2003
mounted an exhibition on this theme.
[6] Beretta 2001, pp. 48-9; Holmes 2003.
[7] Williamson 1851. For reprints of this and
other papers, see Knight 1998, vol. 1.
[8] Hofmann 1862-6, quotation from p. 416.
[9] But see the new book by Klein (2003).
[10] Ellenius 1985, pp. 123, 147-65; Knight
1977, pp. 4, 117.; Desmond 2003, p. 128.
[11] In Ramberg 2003, p. xxi.
References
Accum, Fr.: 1807, System of Theoretical and Practical Chemistry,
2nd ed., Kearsley, London.
Accum, Fr.: 1828, Chemical Re-agents, or Tests, ed. William Maugham,
Tilt, London.
Anderson, W.: 1984, Between the Library and the Laboratory: the Language
of Chemistry in Eighteenth-century France, Johns Hopkins UP, Baltimore
MD.
Armstrong, K.: 1999, A History of God, Vintage, London.
Beretta, M.: 1993, ‘The Role of Symbolism from Alchemy to Chemistry’,
in: R. Mazzolini (ed.), Non-verbal Communication in Science prior to
1900, Olschki, Firenze, pp. 297-319.
Beretta, M.: 2001, Imaging a Career in Science: the Iconography of
Antoine Laurent Lavoisier, Science History, Canton MA.
Berthollet, Cl.L.: 1803, Essai sur la Statique Chimique, Firmin
Didot, Paris.
Berthollet, Cl.L.: 1804, Researches into the Laws of Chemical Affinity,
trans. by M. Farrell, Murray, London (reprinted: Routledge/Thoemmes, London,
1998).
Berthollet, Cl.L.: 1809, Researches into the Laws of Chemical Affinity,
trans.
M. Farrell, Nicklin, Baltimore, MD (reprinted: Da Capo, New York, 1966).
Berzelius, J.J.: 1845, The Use of the Blowpipe in Chemistry and Mineralogy,
trans. by J.D. Whitney, Ticknor, Boston MA (reprinted: Routledge, London,
1998).
Brande, W.Th.: 1830, A Manual of Chemistry: containing the Principal
Facts of the Science, arranged in the Order in which they are Discussed
and Illustrated in the Lectures at the Royal Institution of Great Britain,
3rd ed., Murray, London.
Brock, W.H.: 1992, Fontana History of Chemistry, Fontana, London.
Brock. W.H. (ed.): 1967, The Atomic Debates, Leicester UP, Leicester.
Chaouli, M: 2002, The Laboratory of Poetry: Chemistry and Poetics
in the Work of Friedrich Schlegel, Johns Hopkins UP, Baltimore MD.
Coleridge, S.T.: 1969, The Friend, ed. by B. Rooke, Routledge,
London, vol.1.
Crosland, M.: 1967, The Society of Arcueil, Heinemann, London,
1967.
Crosland, M.P.: 1978, Historical Studies in the Language of Chemistry,
2nd ed, Dover, New York.
Daniell, J.Fr.: 1839, An Introduction to the Study of Chemical Philosophy:
being a Preparatory View of the Forces which Concur to the Production of
Chemical Phenomena, Parker, London.
Davy, H.: 1830, Consolations in Travel: or the Last Days of a Philosopher,
Murray, London.
Davy, H.: 1839-40, Collected Works, ed. by J. Davy, Smith Elder,
London (reprinted: Thoemmes, Bristol, 2001).
Desmond, R.: 2003, Great Natural History Books and their Creators,
British Library, London.
Donovan, A.: 1993, Antoine Lavoisier: Science, Administration and
Revolution, Blackwell, Oxford.
Duncan, A.: 1996, Laws and Order in Eighteenth-century Chemistry,
Oxford UP, Oxford.
Ellenius, A. (ed.): 1985, The Natural Sciences and the Arts,
Almqvist & Wiksell, Uppsala.
Faraday, M.: 1842, Chemical Manipulation: being Instructions to Students
in Chemistry on the Methods of Performing Experiments of Demonstration
or Research, with Accuracy and Success, 3rd
ed., Murray, London (reprinted: Routledge, London, 1998).
Fullmer, J.Z.: 2000, Young Humphry Davy; the Making of an Experimental
Chemist, American Philosophical Society, Philadelphia PA.
Gascoigne, J.: 1998, Science in the Service of Empire: Joseph Banks,
the British State and the Uses of Science in the Age of Revolution,
Cambridge UP, Cambridge.
Goethe, J.W.: 1971, Elective Affinities, trans. by R.J. Hollingdale,
Penguin, London.
Hargittai, I.: 2003, Candid Science III: Conversations with Famous
Chemists, Imperial College, London.
Henry, W.: 1803, An Epitome of Chemistry, 3rd
ed, Johnson, London.
Hoffmann, R. & Torrence, V.: 1993, Chemistry Imagined: Reflections
on Science, Smithsonian, Washington DC.
Hofmann, A.: 1862, ‘On Mauve and Magenta’, Proceedings of the Royal
Institution, 3, 468-83.
Hofmann, A.: 1862-6, ‘On the Combining Power of Atoms’, Proceedings
of the Royal Institution, 4, 401-30.
Holmes, F.L.: 2003, ‘Lavoisier’, in: J.L. Heilbron et al. (eds.),
The
Oxford Companion to the History of Modern Science, Oxford UP, Oxford,
pp. 453-5.
Jackson, Chr. E.: 1975, Bird Illustrators: some Artists in early
Lithography, Witherby, London.
Klein, U.: 2003, Experiments, Models, Paper Tools: Cultures of Organic
Chemistry in the Nineteenth Century, Stanford UP, Stanford CA.
Knight, D. (ed.): 1998, The Development of Chemistry 1789-1914,
Routledge, London.
Knight, D.: 1977, Zoological Illustration, Dawson, Folkestone.
Knight, D.: 2000, ‘Why is Science so Macho?’, Philosophical Writings,
14,
59-71.
Knight, D.: 2002, ‘Scientific Lectures: a History of Performance’, Interdisciplinary
Science Reviews, 27, 1-8.
Knight, D.: 2003, Science and Spirituality: the Volatile Connection,
Routledge, London.
Lavoisier, A.L.: 1970, Elements of Chemistry, trans. by R. Kerr,
William Creech, Edinburgh.
Levere, T.: 1981, Poetry Realized in Nature: Samuel Taylor Coleridge
and Early Nineteenth-century Science, Cambridge UP, Cambridge.
Little, W. et al. (eds.): 1964, The Shorter Oxford English
Dictionary, 3rd ed., Oxford UP, Oxford.
Lyell, Ch.: 1830-3, Principles of Geology: being an Attempt to Explain
the Former Changes of the Earth’s Surface by Reference to Causes now in
Operation, Murray, London.
Mackenzie, C.: 1822, One Thousand Experiments in Chemistry: with
Illustrations of Natural Phenomena; and Practical Observations on the manufacturing
and Chemical Processes at Present pursued in the Successful Cultivation
of the Useful Arts, Phillips, London.
Marcet, J.: 1828, Conversations on Chemistry: in which the Elements
of that Science are Familiarly Explained and Illustrated by Experiments,
11th ed., Longman, London.
Nicholson, W.: 1808, A Dictionary of Practical and Theoretical Chemistry,
Phillips, London.
Nickon, A. & Silversmith, E.: 1987, Organic Chemistry: the Name
Game, Pergamon, Oxford.
Odling, W.: 1870-2, ‘On the Revived Theory of Phlogiston’, Proceedings
of the Royal Institution, 6, 315-25.
Parkinson, J.: 1801, The Chemical Pocket-book: or memoranda Chemica:
arranged as a Compendium of Chemistry: with Tables of Attractions, &c.
Calculated as well for the Occasional Reference of the Professional Student,
as to Supply Others with a General Knowledge of Chemistry, 2nd
ed., Whittingham, London.
Poirier, J.-P.: 1996, Lavoisier: Chemist, Biologist, Economist,
trans. Rebecca Balinski, PENN, Philadelphia PA.
Principe, L. & De Witt, L.: 2002, Transmutations: Alchemy in
Art, Chemical Heritage Foundation, Philadelphia PA.
Ramberg, P.J.: 2003, Chemical Structure, Spatial Arrangement: the
early History of Stereochemistry, 1874-1914, Ashgate, Aldershot.
Scoffern, J.: 1852, Elementary Chemistry: the Circle of the Sciences,
vol. 6, Griffin, Bohn, London (published ca. 1852).
Shelley, M.: 1994, Frankenstein: or the Modern Prometheus, [1818],
afterword by Joyce Carol Oates, University of California, Berkeley (also
ed. by D.L. Macdonald and K.Scherf, 2nd
ed., Broadview, Peterborough, Ontario, 1999).
Smith, J.: 1994, Fact and Feeling: Baconian Science and the 19th-century
Literary Imagination, University of Wisconsin Press, Madison.
Uglow, J.: 2002, The Lunar Men: the Friends who made the Future,
Faber, London.
Vertesi, J.: ‘Light and Enlightenment in Joseph Wright of Derby’s »The
Alchemist«’, http://www.geocities.com/jvertesi/wright/
Williamson, A.: 1851, ‘Suggestions for the Dynamics of Chemistry derived
from the Theory of Etherification’, Proceedings of the Royal Institution,
1,
90-4.
David Knight:
Department of Philosophy, University of Durham, 50, Old Elvet, Durham DH1
3HN, U.K.; D.M.Knight@durham.ac.uk
Copyright Ó
2003 by HYLE and David Knight
|