Abstract: In this paper I explore the character and role of stereoformulas and models of the atom that appeared in the early history of stereochemistry, including those of Jacobus Henricus van’t Hoff, Aemilius Wunderlich, Johannes Wislicenus, Victor Meyer, Arthur Hantzsch, Alfred Werner, and Hermann Sachse. I argue that stereochemists constructed and used stereoformulas in a pragmatic way that ignored the physical implications of the spatial distribution of valence, and that the models of the atom were created to reconcile the physically curious concept of valence with known physical laws. Although such models were explanatory at a deeper level, they had little impact on the theory and practice of chemistry, and were not serious attempts to reduce chemical theory to physical laws.
Keywords: atomic models in 19th century chemistry, stereochemistry, affinity, pragmatism, reduction.
Both of these ubiquitous forms of modeling – physical and symbolic – have their origins in the nineteenth century, when chemists carefully crafted a means of making atoms and molecules anschaulich. Techniques for modeling molecules and atoms date at least to John Dalton’s wooden spheres and graphic circles of the early nineteenth century, and by the 1850s and 1860s, chemists had created a vast array of physical lecture demonstration models and two-dimensional symbolic representations of molecules, particularly in organic chemistry. Curiously, these models were not originally meant to make molecules anschaulich in a physical sense, and all but ignored the structure of the atom itself.[2],[3] Beginning with van’t Hoff’s theory of the tetrahedral carbon atom in 1874, however, chemists began to give those same formulas and models a true physical Anschaulichkeit, and by the end of the nineteenth century, the transformation in meaning had largely been completed. In the words of the chemist-philosopher Roald Hoffmann, chemical models before and after 1874 were the same and yet not the same.[4]
Although the adoption of van’t Hoff’s principles was initially slow,
by the late 1880s and 1890s, the chemical literature blossomed with a new
symbolic language for the three-dimensional properties of molecules (hereafter
called stereoformulas).[5] As I will argue below, chemists
employed stereoformulas to achieve the two traditional aims of nineteenth
century chemistry – explaining the existence of isomers and predicting
the existence of new compounds – even though these formulas were curious,
or even absurd, from the standpoint of physical laws. It is not surprising
then, that concurrent with the appearance of these stereoformulas was the
publication by several stereochemists of unprecedented atomic models that
attempted to provide a sound basis for the physical nature of valence,
bonding and affinity. These atomic models served well as explanatory devices,
but they were not serious attempts to reduce chemistry to physics, and
equally did not further the aims of chemistry.


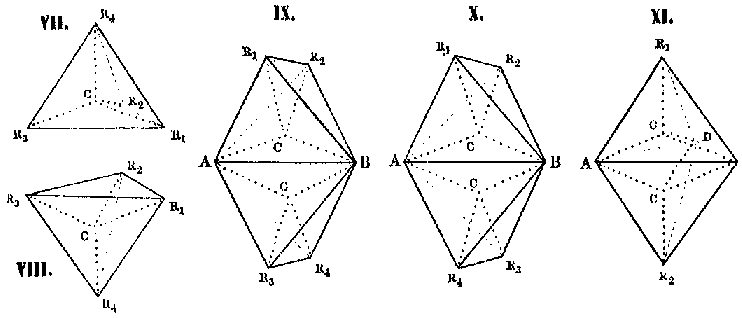
Many subsequent stereoformulas drew on van’t Hoff’s explicit portrayal
of the tetrahedron. In his influential 1887 theoretical essay on the unsaturated
acids and supporting publications, Johannes Wislicenus depicted the tetrahedron
literally, and removed the central carbon atom and valence lines (Fig.
3a).[9] In Arthur Hantzsch and Alfred Werner’s 1890 paper
outlining the stereochemistry of nitrogen, the tetrahedron outwardly resembles
van’t Hoff’s picture (Fig. 3b), yet the nitrogen occupies one vertex and
the edges depict the lines of valence, and not the edges of an imaginary
tetrahedron. Hantzsch would use the same form of representation in his
later papers (Fig. 4a), although he often published papers with no drawings
of the tetrahedron (Fig. 4b), in which the spatial relationships were implied.
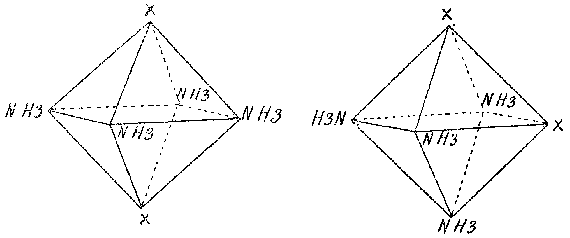
In his ‘Beitrag zur Constitution anorganischer Verbindungen’, Alfred Werner
explained the isomerism of the cobalt ammines by an octahedral arrangement
of groups around the central metal atom (Fig. 5). Because Werner ignored
the individual valences and central atom of the octahedron, his drawings
of the octahedron closely resemble Wislicenus’ drawings.
 |
 |
|
|
|
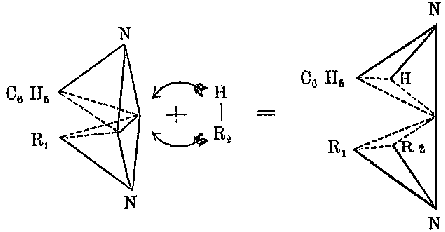 4a
4a
 4b
4b
Figure 4: a) Hantzsch’s use of stereoformulas. This diagram represents an addition reaction to the nitrogen-nitrogen double bond in a diazo compound. From ‘Zur Statik und Dynamik der Stickstoffverbindungen’, in: Festschrift der Naturforschenden Gesellschaft in Zürich 1746-1896, Zürcher and Furrer, Zürich, 1896, pp. 186-202. b) Hantzsch’s depiction of isomeric diazo compounds without explicit stereoformulas. From Hantzsch, ‘Über stereoisomere Diazoamidoverbindungen’, Berichte der deutschen chemischen Gesellschaft, 27 (1894), 1857-67.
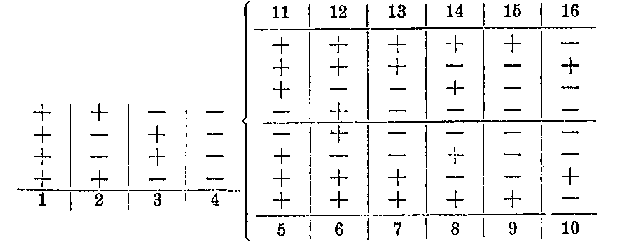
Because of these curiosities, the stereoformulas using literal polyhedra were not universally adopted. Victor Meyer recognized the physical absurdity in these drawings of tetrahedra and chose instead to emphasize the lines of valence in his stereoformulas (Fig. 6). These drawings, Meyer said, "appear to me more convenient than the complicated drawings used by van’t Hoff and Wislicenus, in which the four valences of the carbon are not drawn at all, but instead six tetrahedral edges that of course have no importance."[10] In his investigation of the isomers of glucose, Emil Fischer did not employ the polyhedral stereoformulas, but for purely practical reasons. Because glucose and its isomers possessed four asymmetric carbon atoms, representing them by two-dimensional drawings of tetrahedra would be difficult to realize and visualize. In 1877, van’t Hoff had already proposed a convention for designating the configuration of compounds with multiple asymmetric carbon atoms (Fig. 7). Fischer initially adopted this scheme, but quickly abandoned it in favor of his new Projektionen (the famous Fischer projections), that showed the groups around each asymmetric carbon atom (Fig. 8).[11] Fischer’s stereoformulas did not depict tetrahedra at all; rather, he represented the three dimensionality of each asymmetric carbon atom by means of a two-dimensional convention.
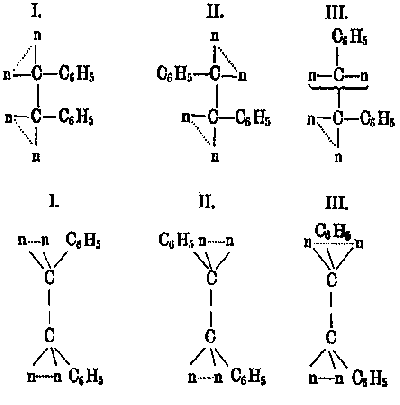
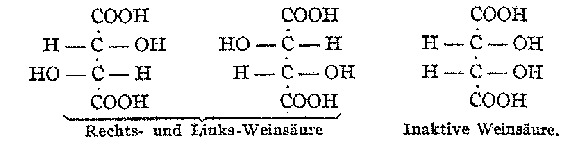
Chemists addressed this problem with varying degrees of sophistication. The easiest solution, adopted by van’t Hoff, Hantzsch, and Fischer, was to ignore it. Van’t Hoff implicitly raised the question of valence when he introduced the tetrahedron, but he was nearly silent about the exact nature of the carbon atom. His only suggestion about the physical location of the affinity unit was in a public letter to the Dutch physicist C.H.D. Buys-Ballot, in which he located the sites of bonding with the faces and not the corners of the tetrahedron.[15] He also later confided to Wilhelm Ostwald that the carbon atom "must consist of tetrahedral symmetry". It seems clear, however, that what he meant by the "tetrahedral carbon atom" was not the atom itself, but the spatial distribution of valences around the atom.[16] The arrangement was defined only in reference to the atoms bound to carbon, and not in the atom itself. That he used different models to emphasize different aspects of his theory illustrates a pragmatic use of stereoformulas without an explicit interest in the actual appearance of the carbon atom.
Hantzsch also practiced stereochemistry without any model of the atom beyond the simple tetrahedral arrangement of atoms. In his 1893 monograph on stereochemistry, Grundriß der Stereochemie, Hantzsch explicitly denied that any kind of theory of valence was necessary for, or followed from, the practice of stereochemistry:
While van’t Hoff, Hantzsch, and Fischer ignored the physical problem of valence, other stereochemists attempted, with varying degrees of sophistication to reconcile it with known physical laws. We can recount here the models offered by Wislicenus, Meyer, Aemilius Wunderlich, Werner, and Hermann Sachse. In 1888, Wislicenus went slightly beyond the pragmatism of van’t Hoff, Hantzsch, and Fischer, and speculated on the nature of the carbon atom in a short reply to a critique of van’t Hoff’s theory by Wilhelm Lossen.[19] Lossen clearly recognized the physical problems with van’t Hoff’s model for the double bond, noting that it was physically impossible because the lines of bonding did not lie on a straight line between carbon atoms. It implied that multivalent atoms were three-dimensional objects with distinct parts, and Lossen doubted the ability of chemists to know anything about these parts. Wislicenus answered Lossen quite directly – it was impossible, he said, not to conceive of atoms as "spatial objects" with their affinities located in different areas of those objects.
Significantly, Wislicenus considered van’t Hoff’s theory as an important milestone in the development of the atomic theory. In his response to Lossen, he remarked that the study of chemical reactions had largely demonstrated the existence of atoms and that the same process would yield information about the parts of those atoms. Because van’t Hoff’s theory provided a model for the structure of the atom, i.e. the direction of valences and possibly the atomic form, it was precisely this theory that would give information about the parts of the atoms. The establishment of configurational formulas led precisely to a deeper understanding of atoms and furthermore, to subatomic structure.[21] In his various public lectures and addresses, Wislicenus made it clear that he regarded the atomic theory as the foundation of chemistry. Without atomism, he said, "the individual pieces of chemical knowledge would be a desolate pile of unrelated and incomprehensible observations, indeed, it would be less than that: to a great extent, it would not exist at all."[22]
While atomism was also important to Meyer, the principles of stereochemistry were more significant for an understanding of valence. In a short theoretical article entitled ‘On Carbon’s Valence and its Bonding Ability’ that appeared in 1876, Meyer had remarked that despite the dazzling success of the structure theory in ordering organic compounds and explaining isomerism, "[…] at the moment, the nature of that what we call a valence or affinity is still completely unclear." The more the theory of valence proved its value, said Meyer, the more one needed a "certain, physically permissible conception" of valence (bestimmte, physikalisch zulässige Vorstellung).[23] Meyer attempted to form a vague idea of the nature of valence by studying the limitations on the carbon atom’s bonding ability. For example, he pointed out that all reactions in which he expected cyclopropanes (three membered rings) as the product gave only products with other structures. The fact that these compounds did not exist – coupled with the non-existence of C2 (carbon with a ‘quadruple’ bond), despite the fact that these compounds were "easily expressable by our usual formulas" – indicated specific limits on the nature of valence.
Meyer became reinterested in the nature of valence in 1887 when he read Wislicenus’ extensive essay on the stereoisomerism of the unsaturated acids, and was inspired to reinvestigate the chemistry of isomeric benzildioximes discovered in his laboratory some years earlier. In 1888, Meyer and his assistant Karl von Auwers published a lengthy article in which they established the stereoisomerism of the benzildioximes and explained this isomerism by proposing that carbon-carbon single bonds, like double bonds, could also have restricted rotation. By an analysis using conventional chemical formulas, Meyer and Auwers predicted the existence of a third isomer of benzildioxime that they isolated in 1889. Meyer and Auwers’ theory of the benzildioximes was clearly inspired and shaped by Wislicenus’ study of the unsaturated acids, but Meyer distanced himself from the physical implications of van’t Hoff’s and Wislicenus’ stereoformulas. In a paper that appeared shortly before the paper on the benzildioximes, Meyer noted that van’t Hoff’s theory of double and triple bonds explicitly raised the question about the physical nature of valence:
Meyer wrote to his brother Richard in early 1888 about his theory of the benzildioximes, expressing excitement about the opportunity this chemistry offered for a more detailed picture of atoms and valence.
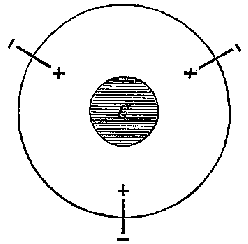
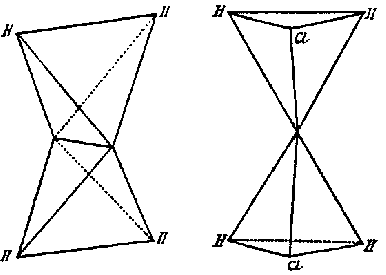
9a |
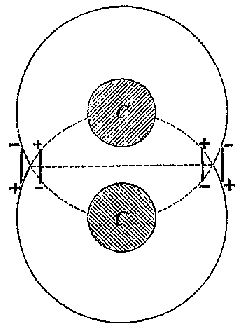
9b |
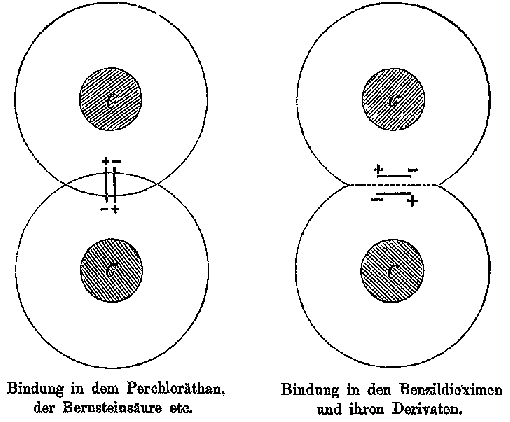
9c |
|
This model presented a strikingly different and more detailed view of the carbon atom than Wislicenus had offered to Lossen one month earlier. Unlike Wislicenus, Meyer and Riecke offered a specific depiction of the parts of the atom and how they worked together to produce the chemical effects that Meyer and Auwers had found. It offered a higher level explanation for the conclusions of stereochemical theory. Why did some atoms combine to allow free rotation and others not? Why were the valences arranged in a tetrahedron? The plastic nature of the dipoles offered a clear picture of how the carbon atom could distort when it was attached to different radicals. An anonymous writer for Nature commented that the theory offered a "strikingly natural explanation [...] of the nature of single, double, and triple linking of carbon atoms".[31]
In 1886, Aemilius Wunderlich, a recently promoted organic chemist at the University of Würzburg, published a novel theory about the form of the carbon atom in a 32 page pamphlet entitled Configuration organischer Moleküle.[32] In a somewhat complicated argument without diagrams, Wunderlich proposed that each atom possessed "binding points" (Bindestellen) that contain a center of bonding, a Bindeschwerpunkt. By chemical and geometrical reasoning, Wunderlich suggested that the carbon atom had a tetrahedral arrangement of Bindestellen (Fig. 10). A single bond would result if the Bindestellen from two carbon atoms joined, but in multiple bonds, it was physically impossible for more than one Bindestelle to join completely. A double or triple bond would therefore occur when two or three Bindestellen got as close as physically possible to each other. The resulting double and triple bonds would then, as was well known, not simply be two or three times as strong as a single bond. Wunderlich’s model for bonding was comprehensive, providing physical models not only for the tetrahedron, but also for the process of addition and elimination reactions, Baeyer’s strain theory, and Kekulé’s oscillation hypothesis for the benzene molecule. Like the model of Meyer and Riecke, the atomic model given in Configuration accounted for the known characteristics of the chemical bond, and was compatible with known physical forces; that is, all attractive forces between atoms were in straight lines. Although it is now obscure, Wunderlich’s model was cited frequently in the chemical literature throughout the 1890s as a physically pleasing conception of valence and bonding.
The last model we will consider was presented by Hermann Sachse in four articles that appeared between 1888 and 1892. In an 1888 article on the configuration of benzene, Sachse attempted to create a stereochemical model of the benzene molecule that was compatible with Kekulé’s cyclohexatriene structure. Sachse agreed with Wislicenus that the carbon atom had a tetrahedral shape and was explicit that the sites of affinity were at the corners:
Except for their novel stereochemical models of benzene and cyclohexane, these two articles would not strike the reader as out of the ordinary, but in two later articles, both published in the Zeitschrift für physikalische Chemie, Sachse provided a deep geometrical and physical explanation for his earlier qualitative statements about the tetrahedral carbon atom. The first article was an expansion of the 1890 article on the configuration of cyclohexane that contained a lengthy, intense mathematical argument for the two configurations of cyclohexane.[37] In the second, which appeared in 1892 shortly before his premature death at the age of 31, Sachse offered "An Interpretation of Affinity". In this 35 page article, Sachse expanded on his earlier brief statements about the form of the carbon atom, and offered a model for bonding that employed two fundamental properties of matter. First, all matter was to a certain extent magnetic and consisted of a "system of smallest particles, around which or in which solenoids stream in circular paths."[38] Second, when the distance between two bodies becomes sufficiently small, attractive forces generally turn into repulsive forces, an "idea that, to my knowledge, molecular physics can scarcely do without". These two principles were then combined with the "fact" of the tetrahedron:
Now if we think of this tetrahedral system as somehow filled with matter, according to the description above it would receive the capacity [Eigenschaft] for exerting a repulsion on other similar systems that find themselves, within certain limits, in its vicinity.[39]
The use of stereoformulas illustrates a general tendency of chemists to be pragmatic, in the simple sense of being practical, in adopting the tools and concepts necessary to reach their goals. That is, chemists will adopt useful concepts and tools even if those tools and concepts raised significant physical or philosophical questions. For example, chemists adopted the principle of valence almost without question, even though it raised crucial physical questions about its nature, because it helped to explain chemical behavior of substances and the appearance of isomers. Chemists had a similar pragmatic attitude towards the use of the atomic theory, by excluding questions about the actual reality of atoms from their discussion, and simply proceeding to use them as if they existed.[41] Chemists constructed and used stereoformulas in a similar pragmatic way. Stereoformulas were devised to make anschaulich the explanation of isomers by the tetrahedral carbon atom or octahedral metal atom, and were tools for portraying visually what could not be described verbally. Stereochemists used stereoformulas as if the tetrahedron were real, despite the fact that it forced questions about the nature of affinity and valence – questions that could be answered, but did not require an answer. We must also keep in mind that because these representations were meant to differentiate isomers by showing spatial differences in molecules, stereoformulas did not represent the three-dimensional characteristics of the atom. Therefore, a specific model for the carbon atom was unnecessary, as Fischer and Hantzsch (and to a large extent, Wislicenus) carried out highly successful research programs without addressing the nature of valence.
If atomic models were unnecessary for the success of stereochemistry, then what role did they play in the thought of nineteenth century chemists? In simple terms, they served to explain the phenomena of valence, bonding, and the tetrahedron at a higher level. But while they were explanatory (with varying degrees of success), they were not at all predictive for chemical theory. The models they presented were ‘stories’ meant simply to account for the physical characteristics of the carbon atom demanded by stereochemical theories. They were independent of chemical theory and irrelevant to the ‘progress’ of stereochemistry, that is, its capability of predicting isomers or postulating reaction mechanisms. Even the most influential of the models, those by Wunderlich and Werner, remained purely explanatory and had no predictive character. The concept of affinity in both was still rather vague physically, as it remained an undefined attractive force. Sachse’s model had the physically most sophisticated conception of valence and affinity, but his model was also an attempt to explain in more fundamental terms what stereochemists had already accepted: that the carbon atom was tetrahedral, that in carbon-carbon single bonds there was free rotation, and that in carbon-carbon double bonds there was no free rotation.
The pragmatic use of stereoformulas and the independence of atomic models from chemical theory are also reinforced by doubts that chemists expressed about ever understanding the ultimate nature of matter. In a letter to Arrhenius, van’t Hoff noted the provisionary nature of the tetrahedron:
Yet in describing the use of stereoformulas and atoms as ‘pragmatic’ or ‘practical’, we must be careful not to equate this pragmatism with pure instrumentalism. ‘Pragmatism’ in the sense described here does not mean, for example, that chemists considered stereoformulas as merely instruments that did not depict reality independent of human experience. Stereochemists believed that the groups around the carbon atom were arranged in a tetrahedron, and that stereoformulas represented in some fashion the molecule as a physical object. In short, they believed they had access to the physical appearance of the molecule, and had not simply invented instruments for prediction. Furthermore, as Wislicenus made evident, considering molecules as physical objects – and thereby implying a three-dimensional distribution of valences of the atoms in the molecule – necessitated considering the atom’s properties as a physical object, specifically how the valences could be directed in space. Stereochemists therefore created the first plausible epistemological foundation for a true physical atomism in organic chemistry, threatened by the quasi-mechanical formulas of the structure theory.[47] Describing chemists’ use of stereoformulas as pragmatic therefore does not preclude their belief in the reality of the spatial arrangement of valences, nor the related conviction that they could address – as some did – and eventually solve the theoretical problems entailed by the spatial division of affinity.
The pragmatic use of stereoformulas and the simultaneous appearance of reductionist models of the atom also beautifully illustrate the tension in nineteenth century chemistry between chemical and physical explanations. During much of the nineteenth century, chemists created a largely autonomous and non-mathematical discipline with unique ontological, epistemological, and methodological characteristics. Yet chemists always had the lurking conviction that chemistry would only become a ‘true’ science when it had been reduced to physical laws described by mathematics, and chemists therefore felt compelled to suggest a physical basis for chemical theories.[48] In his ‘Deutung der Affinität’, Sachse employed extensively "the language of mechanics, in which ultimately shall just dissolve (sich auflösen) the language of our science."[49]
Meyer exemplified this tension between autonomy and reduction when he recognized a need for a physical conception of valence, but was reluctant to endorse fully his own theory of the atom that met this need. He also revealed this tension in his 1889 lecture ‘Contemporary Chemical Problems’, given in a general session of the Heidelberg meeting of the Gesellschaft deutscher Naturforscher und Ärzte. Meyer placed chemical theory in a peculiar, almost schizophrenic, position. He appeared to oscillate between two poles, at first advocating the reduction of chemical theory to mathematical physics and then advocating its theoretical autonomy. The final goal of chemical theory, according to Meyer, was a complete reduction of chemical reactions to mathematical mechanics, because "nature is not understood until we are able to reduce its phenomena to simple movements, mathematically traceable".[50] He was certain that all chemical explanations would one day be completely understood in mathematical terms.
According to Meyer, the "infancy" of chemistry was not a "blemish", nor did it detract from the "immense achievements [it had] registered on its own."[53] The lack of rigorous mathematical principles, Meyer claimed, gave chemical thought a plasticity that the logical, mathematical sciences lacked. Chemists had a greater tendency for imaginative thought or speculation (Phantasie) that brought a creative enjoyment similar to that experienced by artists.
This reluctance towards complete reduction and the pragmatic use of
chemical formulas has never really disappeared. The concepts of hybridization
and molecular orbitals, like their nineteenth century counterparts, offer
a deeper physical explanation for the appearance of valence, bonding, and
their three-dimensional arrangement. They are much more sophisticated in
that they allow predictions about spectroscopic properties (and to a limited
extent chemical reactivity), but they are no more predictive than their
nineteenth century equivalents about the number of possible isomers than
non-mathematical structural and stereoformulas. The uniquely chemical concept
of isomerism, developed to explain the existence of and differences between
specific kinds of substances, is therefore not necessarily reducible to
mathematics. As any first year student in organic chemistry learns, there
is no mathematical formula for deriving all possible structures and stereoisomers
for all possible isomers from a given compositional formula.[56]
It often amazes students of modern chemistry that the tetrahedral carbon
atom predates electronic theories of bonding by fifty years, and even the
electron itself by twenty five years. Like its nineteenth century counterparts,
Linus Pauling’s mathematical model of orbital hybridization was created
to explain what was already well known at a scientifically ‘lower’ level
for over fifty years: that the carbon atom was tetrahedral.[57]
[1] E. Francoeur, ‘The Forgotten Tool: the Design and Use of Molecular Models’, Social Studies of Science, 27 (1997), 7-40, and ‘Beyond Dematerialization and Inscription: Does the Materiality of Molecular Models Really Matter?’, this issue of HYLE. I thank Francoeur for making the unpublished manuscript available to me.[2] Those models that did imply an atomic structure, such as Kekulé’s famous ‘sausage formulas’, were used heuristically, and not meant to be a literal snapshot of the atom.
[3] A.J. Rocke, Chemical Atomism in the Nineteenth Century: From Dalton to Canizarro, Ohio State University Press, Columbus, 1984; Ch. Meinel, ‘Modeling a Visual Language for Chemistry, 1860 – 1875’, unpublished manuscript. I thank Dr. Meinel for making his manuscript available to me.
[4] R. Hoffmann, The Same and Not the Same, Columbia Univ. Pr., New York, 1995.
[5] The term ‘stereoformulas’ was not used until after 1889, after Victor Meyer introduced the word ‘stereochemistry’. The prefix ‘stereo-’ subsequently was employed frequently to distinguish structural features (connectivity) from spatial features of molecules. Describing chemical formulas published before 1889 as ‘stereoformulas’ is therefore somewhat anachronistic, but for the purposes of this article, I have chosen to use it for the sake of clarity and consistency, rather than historical accuracy.
[6] The other co-founder of stereochemistry, Joseph Achille Le Bel, did not portray graphically the tetrahedron, and did not use stereoformulas. J.H. van’t Hoff, Voorstel tot Uitbreiding der tegenwoordig in de scheikunde gebruikte Structuur-Formules in de ruimte; benevens een daarmeê samenhangende opmerkung omtrent het verband tusschen optisch actief Vermogen en Chemische Constitutie van Organische Verbindingen, Greven, Utrecht, 1874; La chimie dans l’espace, Bazendijk, Rotterdam, 1875; and Die Lagerung der Atome in Raume, Vieweg, Braunschweig, 1877.
[7] Kekulé developed these models to illustrate multiple bonds, but like most chemists who developed such models, he did not give the arrangement of valences any physical significance. Meinel, ‘Visual Language’ (Note 3); O.B. Ramsay, ‘Molecular Models in the Early Development of Stereochemistry: I. The van’t Hoff Model’ & ‘II. The Kekulé Models and the Baeyer Strain Theory’, in: O.B. Ramsey (ed.), Van’t Hoff Le Bel Centennial, American Chemical Society, Washington, DC, 1974, pp. 74-96.
[8] Van’t Hoff, Die Lagerung (Note 6), p. 47; O.B. Ramsay, ‘Molecular Models’ (Note 7).
[9] J. Wislicenus, ‘Ueber die räumliche Anordnung der Atome in organischen Molekülen und ihre Bestimmung in geometrisch-isomeren ungesättigten Verbindungen’, Abhandlungen der mathematischen-physikalischen Classe der königlichen sächsischen Gesellschaft der Wissenschaften, 14 (1887), p. 1.
[10] "Diese Bezeichnungsweise scheint mir zweckmässiger als die von van’t Hoff und Wislicenus gebrauchten complicirten Zeichnungen, bei welchen die vier Valenzen des Kohlenstoffs, auf welche es gerade ankommt, gar nicht gezeichnet sind, statt ihrer aber sechs Tetraëder-Kanten, welche doch für die Betrachtung ohne jeden Belang sind." (V. Meyer & K. von Auwers, ‘Untersuchungen über die zweite van’t Hoff’sche Hypothese’, Berichte der deutschen chemischen Gesellschaft, 21 (1888), pp. 790-817 [786]). Unless noted, all translations are mine.
[11] Fischer describes the invention of these projections from Gummimodellen, physical molecular models developed by Friedländer, at the Karlsruhe Technische Hochschule. The flat drawings were derived by literally squashing these flexible three-dimensional models into two dimensions. E. Fischer, ‘Über die Konfiguration des Traubenzuckers und seiner Isomeren’, I & II, Berichte der deutschen chemischen Gesellschaft, 24 (1891), 1836ff., 2683ff. (repr. in: Emil Fischer, Gesammelte Werke, ed. by M. Bergmann, Springer, Berlin, 1906-1923, vol. 5, pp. 417-27, 427-31).
[12] M.J. Nye, ‘Explanation and Convention in Nineteenth-Century Chemistry’, in: R.P.W. Visser (ed.), New Trends in the History of Science, Editions Rodopi, Amsterdam, 1989, pp. 171–86.
[13] A.J. Rocke, ‘Convention Versus Ontology in Nineteenth-Century Organic Chemistry’, in: J.G. Traynham (ed.), Essays on the History of Organic Chemistry, Louisiana State University Press, Baton Rouge, 1987, pp. 1–20 (14).
[14] "Daß aber eine solche Kraft sich nicht nach allen Richtungen des Raumes bethätigen soll, sondern nur nach ganz bestimmten, widerspricht dem Begriff einer Anziehungskraft." (K. von Auwers, Die Entwickelung der Stereochemie, Carl Winter’s Universitätsbuchhandlung, Heidelberg, 1890, p. 25).
[15] J.H. van’t Hoff, ‘Isomerie en atoomligging. Antwoord op den openbaren brief van Dr. C.H.D. Buys Ballot’, Mandblad vor Natuurwetenschappen, 6 (1875), pp. 37ff.; German translation by E. Cohen in: Jacobus Henricus van’t Hoff: Sein Leben und Wirken, Akademische Verlagsgesellschaft, Leipzig, 1912, pp. 104–113.
[16] Van’t Hoff to Wilhelm Ostwald, January 20, 1888, in: H.-G. Körber (ed.), Aus dem wissenschaftlicher Briefwechsel Wilhelm Ostwalds, 2 vols., Akademie-Verlag, Berlin, 1969, vol 2, p. 213.
[17] "[die Chemie bedarf] wenigstens in ihrem gegenwärtigen Entwickelungsstadium, keiner bestimmten Vorstellung über Art und Ursache des intramolekularen Zusammenhaltes der Atome, also über die Natur der chemischen Affinität, oder über Art und Ursache des Zahlenverhältnisses, in welchem sich verschiedene Atome verbinden, also über die Natur der Valenz; sie bedarf zur Zeit nur der durch die Existenz der Isomerie überhaupt bewiesenen Vorstellung, daß sich die Atome innerhalb des Molekel nicht in einem chaotischen Zustande, sondern in einer innerhalb gewisser Grenzen stabilen Gleichgewichtslage befinden." (A. Hantzsch, Grundriss der Stereochemie, Trewendt, Breslau, 1893, p. 1).
[18] "Die Hypothese selbst lässt sich […] ganz unabhängig von dem schwankenden Begriffe der Valenz und den noch mehr schwankenden Vorstellungen über ‘Richtung’, ‘Ablenkung’, und ‘Bindung’ der Valenz entwickeln." (A. Hantzsch & A. Werner, ‘Bermerkungen über stereochemisch isomere Stickstoffverbindungen’, Berichte der deutschen chemischen Gesellschaft, 23 (1890), 2764-9 [2769]).
[19] W. Lossen, ‘Ueber die Lage der Atome in Raume’, Berichte der deutschen chemischen Gesellschaft, 20 (1887), 3306–10.
[20] J. Wislicenus, ‘Über die Lage der Atome in Raum. Antwort auf Lossen’s Frage’, Berichte der deutschen chemischen Gesellschaft, 21 (1888), 581-5 (584). A closer analysis of Lossen’s critique and a full translation of Wislicenus’ response is given in P.J. Ramberg, "Johannes Wislicenus, Atomism, and the Philosophy of Chemistry: A Translation and Commentary", Bulletin for the History of Chemistry, 15/16(1994), 45-53.
[21] Wislicenus, ‘Lage der Atome’ (Note 20), p. 582.
[22] "[dann] wäre das chemische Einzelwissen ein wüster Haufen unzusammenhängender und daher unverständlicher Beobachtungen, ja es wäre weniger als das: es wäre zum größten Theile überhaupt nicht vorhanden. […] Für die heutige Chemie, auch wenn sie sich der hypothetischen Natur der atomistischen Anschauung durchaus bewusst bleibt, sind die Elementaratome Realitäten. Obgleich niemand sie sinnlich wahrgenommen hat, noch je wahrnehmen wird, so kennen wir von ihnen gewisse, zum Theil genau gemessene Eigenschaften, […]." (J. Wislicenus, Die Chemie und das Problem von der Materie, Alexander Edelmann Verlag, Leipzig, 1893, pp. 20-1).
[23] "[daß wir uns über] die Natur dessen, was wir eine Valenz oder Verwandschaft nennen, vorläufig noch unvollkommen im Unklaren sind." V. Meyer, ‘Zur Valenz und Verbindungsfähigkeit des Kohlenstoffs’, Justus Liebig’s Annalen der Chemie, 180 (1876), 192-206 (192).
[24] "Sobald man aber die zweite van’t Hoff’sche Hypothese annimmt, […] eine Hypothese, deren fundamentale Bedeutung zuerst Wislicenus erkannt und welche er zur Basis seines Lehrgebäudes genommen und mit bewunderungswürdiger Consequenz durchgeführt hat – müssen bestimmte Vorstellungen über die Form der Atome und die Natur der Valenzen eingeführt werden, wenn die Speculationen nicht der exacten Grundlagen entbehren sollen. Die Annahme an bestimmten Stellen des leeren, von Atomen freien Raum sich treffender Valenzen kann als solche nicht gemacht werden und ist nur auf dem Papier oder am Modell möglich, wo statt der Kräfte (Valenzen) Striche und Drähte figuriren. Jetzt ist es nothwendig – und, wie ich glaube, auch möglich – dass positive und physikalisch haltbare Hypothesen über die Natur der Valenz gemacht und consequent durchgeführt werden." (V. Meyer & R. Demuth, ‘Zur Kenntniss der Isodibrombernsteinsäuren’, Berichte der deutschen chemischen Gesellschaft, 21 (1888), 264-270 [265]).
[25] "Ich bin ganz fabelhaft erregt über all diese Sachen, träume davon und gehe manchmal bei Tage wie im Traume herum. Denn ich habe das Gefühl, daß ein großer Schritt in der Erkenntnis der Natur weiter gemacht wird, wir kriegen doch schon eher Begriffe von Atom und Valenz. Vor allem arbeiten wir nun mit Eifer daran, wirkliche Isomere zu machen, was leider sehr viel technische Schwierigkeiten bietet und nicht so rasch geht. Es ist, wie Hofmann seinerzeit sagte, als er die Phosphoniumbasen voraus ahnte: ‘Doch genug der Theorie! Langsam und nur von ferne folgt der schleppfüßige Versuch dem Fluge leicht beschwingter Phantasie!’" (Victor Meyer to Richard Meyer, 25 Januar 1888, in: R. Meyer, Victor Meyer: Leben und Wirken eines deutschen Chemikers und Naturforschers 1848–1897, Akademische Verlagsgesellschaft, Leipzig, 1917, p. 216.)
[26] W. Voigt, ‘Eduard Riecke als Physiker’, Physikalische Zeitschrift, 16 (1915), 219-21. Pyroelectricity is the ability of some minerals to develop electrical poles when heated.
[27] "[…] jede Valenz als bedingt […] durch eine gewisse Combination zweier entgegengesetzt elektrischer Theilchen" (V. Meyer & E. Riecke, "Einige Bemerkungen über das Kohlenstoffatom und die Valenz," Berichte der deutschen chemischen Gesellschaft, 21 (1888), 946-56 (946).
[28] Anonymous reviews of Meyer’s lecture to the Göttingen Chemical Society in Nature, 37 (1888), 327; and Chemiker-Zeitung, 12 (1888), 140.
[29] "[daß das Atom] umgeben ist von einer Aetherhülle, welche bei einem isolirten Atome, wie diese selbst, kugelförmige Gestalt besitzt; das Atom selbst betrachten wir als den Träger der spec[ifischen] Affinitäten, die Oberfläche der Hülle als den Sitz der Valenzen. Jede Valenz denken wir uns bedingt durch das Vorhandensein zweier entgegengesetzter elektrischer Pole, welche in den Endpunkten im Vergleich zum Durchmesser der Aetherhülle an kleinen geraden Linie befestigt sind." (V. Meyer & E. Riecke, ‘Einige Bemerkungen’ [Note 27], p. 951).
[30] "[Sind] die an den beiden verschiedenen Kohlenstoffatomen haftenden Gruppen sehr verschiedenartiger Natur – üben also die specifischen Affinitäten derselben ihren orientirenden Einfluß in hohem Maasse – so wird ein stabiles Verharren in der weniger begünstigten Lage schwer oder unmöglich sein, und die zweite, freie Rotation ausschließende Form der Bindung wird in der unbegünstigte Lage nicht zu Stande kommen können. Stehen die Gruppe aber in ihren chemischen Functionen, bezw. dem Grade ihrer Negativität (Acidität), einander nahe, so daß die Affinitäten nur schwach orientirend wirken, so wird die Fixirung in beiden Lagen, der begünstigten wie der weniger begünstigten – die sich in diesem Falle in Bezug auf Stabilität nicht allzu sehr von einander unterschieden – möglich sein." (ibid, p. 953-5).
[31] Anonymous, Nature, 37 (1888), 327.
[32] Ae. Wunderlich, Configuration organischer Moleküle, Leitholdt, Würzburg, 1886, p. 1.
[33] A. Werner, ‘Beiträge zur Theorie der Affinität und Valenz’, Vierteljahrschrift der Züricher Naturforschenden Gesellschaft, 36 (1891), 129-69; trans. by G.B. Kauffman, ‘Alfred Werner’s Habilitationsschrift’, Chymia, 12 (1967), 183-216 (191).
[34] Kauffman, ‘Habilitationsschrift’ (Note 33).
[35] "Das Kohlenstoffatom besitzt, gleichviel wie es im übrigen gestaltet sein mag, vier Punkte von grösster gleicher Entfernung vom Mittelpunkte, die in den Ecken eines regulären Tetraëders liegen, und nach denen die vier Affinitätskräfte gerichtet sind. Diese Punkte mögen daher als Affinitätspunkte bezeichnet werden. […] [Es] liegen auch den Atomen der übrigen Elemente gewisse durch Zahl und Lage der Affinitätspunkte bestimmte geometrische Ideale zu Grunde." (H. Sachse, ‘Über die Configuration des Benzolmoleküls’, Berichte der deutschen chemischen Gesellschaft, 21 (1888), 2530-8 [2530]).
[36] H. Sachse, ‘Ueber die geometrischen Isomerien der Hexamethylenederivate’, Berichte der deutschen Chemische Gesellschaft, 23 (1890), 1365–6. This paper is analyzed in C.A. Russell, ‘The Origins of Conformational Analysis’, in: Ramsey, Van’t Hoff Le Bel Centennial (Note 7), pp. 159–178; and O.B. Ramsay, ‘The Early History and Development of Conformational Analysis’, in: Traynham, Essays, (Note 13), pp. 54–77.
[37] H. Sachse, ‘Über die Konfigurationen der Polymethylenringe’, Zeitschrift für physikalische Chemie, 10 (1892), 203-41.
[38] "[…] ein System kleinster Teilchen, um welche oder in welchen Solenoidströme kreisen. […] eine Vorstellung, die die Molekularphysik meines Erachtens kaum entbehren kann. […] Denken wir uns ein reguläres Tetraëder, in welchem eine grosse Anzahl von festen Punkten nach einem bestimmten Gesetz verteilt ist. Diese Punkte seien von kreisformigen elektrischen Strömen umflossen, derart, dass sie die Mittelpunkte dieser Kreise bilden. Während also der Ort dieser Kreismittelpunkte unveränderlich ist, soll die Lage der Kreisebene und die Richtung des Stromes veränderlich sein. Beide werden nur durch den gegenseitigen Einfluss der Ströme aufeinander bestimmt, d. h. nur durch das Gesetz der Anordnung jener festen Punkte. Sobald aber einem solchen System ein Elektromagnet genähert wird, so werden dadurch jene Ströme in bestimmter Weise neu gerichtet. Die Solenoide können wir natürlich, wenn es das Interesse der Anschaulichkeit erfordert, stets durch geradelinige Magneten ersetzt denken, deren Richtung senkrecht auf jener Kreisfläche steht. […] Wenn wir nun dieses tetraëdrische System uns irgendwie mit Materie erfüllt denken, so erhält es gemäss dem oben gesagten die Eigenschaft, auf andere ähnliche Systeme, die sich in seiner Umgebung innerhalb gewisser Grenzen befinden, eine Abstossung auszuüben." (H. Sachse, ‘Eine Deutung der Affinität’, Zeitschrift für Physikalische Chemie, 11 (1893), 185–219 [186-7]).
[40] The idea of ‘isomer counting’ was introduced by G.B. Kauffman, ‘The Discovery of Optically Active Coordination Compounds: A Milestone in Stereochemistry’, Isis, 65 (1973), 38-62.
[41] For the example of atomism, see B. Görs, Chemischer Atomismus: Anwendung, Veränderung, Alternativen im deutschprachigen Raum in der zweiten Hälfte des 19. Jahrhunderts, ERS Verlag, Berlin, 1999.
[42] Van’t Hoff to Svante Arrhenius, undated, quoted by A.G. van Melsen, From Atomos to Atom: A History of the Concept Atom, Harper, New York, 1960.
[43] "[Dies] entsprang lediglich dem Bedürfniss, welches Riecke und ich gleichzeitig empfanden, an Stelle des bisher gänzlich unbestimmten Begriffes der Valenz eine greifbare Vorstellung zu setzen. Dass unsere Hypothese mit gewissen pyroelektischen Erscheinungen sowie mit den Beobachtungen über die Benzildioxime harmonirt, kann derselben in gewissem Sinne als Stütze dienen; allein unrichtig ist die Umkehrung, dass für meine Erklärung der Isomerie der Benzildioxime jene Hypothese über die Valenz nothwendig sei. […] Gegen den Ausspruch von Hantzsch und Werner, dass unsere Auffassung der isomeren Benzildioxime ‘ein neues Princip über das Wesen der Valenz erfordere’, muss ich sonach Verwahrung einlegen." (V. Meyer, ‘Die Ergebnisse und Ziele der Stereochemischen Forschung’, Berichte der deutschen chemischen Gesellschaft, 23 (1890), 567–619 [601]).
[44] "Wie sehr weit der Weg von diesem, heut dargelegten Standpunkte bis zu einer umfassenden physikalischen Theorie der chemischen Erscheinungen noch ist, das empfindet niemand klarer als wir selbst. Möge unsere Untersuchung nur als ein bescheidener Fortschritt auf diesem noch so dunklen Pfade angesehen werden, welcher für einige Erscheinungen ein klareres Verständniß anzubahnen und zunächst zu weiterem Vordringen im Gebiete des Valenzproblems anzuregen bestimmt ist." (Meyer & Riecke, ‘Einige Bemerkungen’ [Note 29], p. 956).
[45] "Über das Wesen der Valenz – über die Ursache, durch welche die verschiedene Sättigungscapacität der Elemente bedingt ist – besitzen wir noch keine klare Vorstellung. Aus den Beobachtungen können wir zunächst nur ableiten, daß ein Kohlenstoffatom eine viermal grössere Atombindkraft als ein Wasserstoffatom besitzt, weil es eben im Stande ist, seine Affinität vier anderen Atomen gegenüber zu äussern." (V. Meyer & P. Jacobsen, Lehrbuch der organische Chemie, 3 vols., Leipzig 1893, vol 1, p. 58).
[46] "[…] wie denkt sich die Chemie das letzte Princip der Materie beschaffen? Kann es der Lichtäther sein, dessen die Physik bedarf um gewisse Gruppen von Erscheinungen, vor allem diejenigen strahlenförmiger Fortpflanzung transversaler Schwingungen zu erklären? Sind es vielleicht Wesenheiten ganz anderer Ordnung als die chemischen Elementaratome, etwa ausdehnungslose, bewegte und auf einander wirkende Kraftcentren, durch deren räumliche Aggregation erst die einfachsten Corpusculareinheiten entstehen? Ist nicht die Materie, der thatsächlich von der Erfahrung geschaffene Begriff, wie alle unsere äussere Erfahrung nur eine Produkt der Wirkungen der im All in unveränderlicher Quantität enthaltenen Energie? Die Chemie als solche hat auf derartige Fragen keine bestimmte Antwort, da alle diese Vorstellungen – wie auch der stofflich gedachte Lichtäther – rein hypothetischer Natur sind." (Wislicenus, Materie, [Note 22], p. 26-27).
[47] Many chemists before the emergence of stereochemistry were physical atomists, but any belief in physical atoms was usually not reflected in the meaning they gave to structural formulas and molecular models. Chemists placed strict ontological and epistemological constraints on the meaning of structural formulas and physical models, interpreting them as ‘reaction formulas’ that did not represent physical objects. Stereochemists, on the other hand, saw structural formulas as representing the physical molecule, and had developed specific ways of justifying this meaning. Cf. P.J. Ramberg, Theory and Methodology in the Early History of Stereochemistry, (PhD: Indiana University, 1993); Rocke, Chemical Atomism (Note 3); Meinel, ‘Visual Language’ (Note 3); U. Klein, Experimente, Modelle, Paper-Tools: Kulturen der organischen Chemie im 19. Jahrhundert, Habilitationsschrift, University of Konstanz, 1999.
[48] The relationship between nineteenth century chemistry and physics is given in M.J. Nye, ‘Physics and Chemistry: Commensurate or Incommensurate Sciences?’, in: M.J. Nye, J.L. Richards, R.H. Stuewer (eds.), The Invention of Physical Science: Intersections of Mathematics, Theology and Natural Philosophy Since the Seventeenth Century, Kluwer, Dordrecht, 1992, pp. 205-224; and in From Chemical Philosophy to Theoretical Chemistry: Dynamics of Matter and Dynamics of Disciplines, 1800-1950, University of California Press, Berkeley, 1993.
[49] "[…] wenn wir uns der Sprache der Mechanik bedienen, in die sich ja die Sprache unserer Wissenschaft schliesslich auflösen soll." (H. Sachse, ‘Affinität’ (Note 38), p. 185).
[50] V. Meyer, Chemische Probleme der Gegenwart; Heidelberg, Carl Winter, 1890. L.H. Friedburg translated the address in 1889, ‘The Chemical Problems of Today’, Journal of the American Chemical Society, 11 (1889), 101-120. I have retranslated the title, but all subsequent translations are Friedburg’s.
[52] Ibid., p. 103. Meyer’s emphasis.
[54] Ibid., p. 103. Meyer’s emphasis.
[56] There is the rule, introduced by van’t Hoff in 1875, that predicts that a compound with n stereocenters, will have a maximum of 2n possible stereoisomers. This maximum number is easily reduced, however, by the presence of meso stereoisomers, making the prediction more difficult. In any case, this formula applies only to stereoisomers. There is no formula for generating the maximum number of structural isomers from a given empirical formula.
[57] B.S. Park, ‘Graphs, Pictures and Diagrams: Visual Representations in the Making of Quantum Chemistry’, paper presented at the Workshop on Types of Paper Tools and Traditions of Representation in the History of Chemistry, Max-Planck-Institute for the History of Science, December 1999.
Peter J. Ramberg:
Max-Planck-Institut für Wissenschaftsgeschichte, Wilhelmstraße 44, D-10117 Berlin, Germany; ramberg@mpiwg-berlin.mpg.de