The Hidden History of Phlogiston
How Philosophical Failure Can Generate Historiographical
Refinement
Hasok Chang*
Abstract: Historians often feel that
standard philosophical doctrines about the
nature and development of science are not adequate for representing the
real history of science. However, when philosophers of science fail to
make sense of certain historical events, it is also possible that there
is something wrong with the standard historical descriptions of those
events, precluding any sensible explanation. If so, philosophical
failure can be useful as a guide for improving historiography, and this
constitutes a significant mode of productive interaction between the
history and the philosophy of science. I illustrate this methodological
claim through the case of the Chemical Revolution. I argue that no
standard philosophical theory of scientific method can explain why
European chemists made a sudden and nearly unanimous switch of
allegiance from the phlogiston theory to Lavoisier’s theory. A careful
re-examination of the history reveals that the shift was neither so
quick nor so unanimous as imagined even by many historians. In closing
I offer brief reflections on how best to explain the general drift
toward Lavoisier’s theory that did take place.
Keywords: Chemical Revolution,
phlogiston, history and philosophy of science, scientific change,
Lavoisier.
1. Introduction
Many historians of science have felt for some
time
that standard philosophical doctrines about the nature and development
of science are not adequate for representing the real history of
science. This is one of the major obstacles standing in the way of
productive collaboration between historians and philosophers of
science. I would like to turn the historians’ frustration on its head:
when philosophers of science fail to make sense of certain historical
events, that may not always be the philosophers’ fault; it is possible
that there is something wrong with the accepted historical descriptions
of those events, precluding any sensible explanation of why they took
place. If that is the case, philosophical failure can serve usefully as
a guide for improving historiography. From this point of view, we can
easily see that historians and philosophers of science will have much
to talk to each other about, because these philosophical failures are
quite abundant!
I will use my current work-in-progress on the
Chemical Revolution in order to illustrate this mode of
history-philosophy interaction[1].
The task at hand is to explain the decisions that scientists made in
the Chemical Revolution. For philosophers and philosophical historians
of science, such explanations need to be given in the framework of some
philosophical theory of scientific method (or rationality, or progress,
or at least something related to the basic nature of science). So, my
thesis of philosophical failure amounts to the claim that no theory of
scientific method has been able to explain the event that most
philosophers of science have understood the Chemical Revolution to be.
In the words of Paul Thagard, this is what the event consisted in:
In 1772, when Lavoisier first began to form
his
views, the dominant theory in chemistry was the phlogiston theory of
Stahl (1723/1730). By 1789, when Lavoisier published his Traité
[…] the vast majority of chemists had gone over to Lavoisier’s oxygen
theory, which gave a very different explanatory account of the
phenomena of combustion, calcination, and respiration […]. [Thagard
1990, p. 184]
Although there is a more sophisticated
historical
literature on the Chemical Revolution that I will be drawing from later
on, the notion of the Chemical Revolution expressed by Thagard is
pretty much the standard view of historians, too – rather than an
instance of the careless and gross distortions of history that one
sometimes finds in the philosophical literature. John McEvoy’s recent
overview of the historiography of the Chemical Revolution notes: "The
Chemical Revolution has generally been regarded as the very paradigm of
a scientific revolution." What a scientific ‘revolution’ means, of
course, is a point of great contention, but McEvoy observes that "the
suddenness, brevity and pace of the Chemical Revolution, together with
the burst of new discoveries and foundational conflicts that
accompanied it, marked it in the minds of many commentators as arguably
the best example of a classic revolution in the history of science."
(McEvoy 2010, pp. 18-19)
Therefore I take it as a broadly accepted
historical view that whatever else the Chemical Revolution was, it
consisted in a rather sudden and nearly unanimous switch of allegiance
by late 18th-century European chemists from the phlogiston theory to
Antoine-Laurent Lavoisier’s ‘anti-phlogistic’ theory. Describing the
failure of philosophers to explain why this abrupt change took place is
the remit of Section 2 below. And then I will move on to use the
philosophical failure historiographically in the way promised above.
That is to say, I will advance a revised description of the Chemical
Revolution in Section 3, followed by an explanation of the newly
described event in Section 4. I will close with some general and
abstract reflections on the history-philosophy relation in Section 5.
2. Philosophical failures in explaining the Chemical
Revolution
2.1 Basic empiricism
Some of the philosophical explanations of the
Chemical Revolution on offer can be disposed of quite easily[2].
Some people think that the phlogiston theory deserved to be consigned
to the dustbin of history because phlogiston was just an imaginary
entity, not based on anything empirical. This is a basic misconception,
as phlogiston had some detailed links with observed phenomena and with
very concrete practical operations. And Lavoisier’s theory relied
essentially on caloric, the material fluid of heat, which was just as
unobservable or hypothetical as phlogiston.
Even many of those who do recognize the
respectable
empirical character of phlogiston think that the phlogiston theory was,
in the end, factually inadequate. As space is limited I will only
discuss the most sophisticated version of this argument known to me,
due to Philip Kitcher. Kitcher (1993, p. 272) sets out to demolish the
view that "there was no cognitively superior reasoning available to the
participants, which would have decided the issue in favor of
Lavoisier". He wishes to "argue that this fashionable picture is a
myth" and in fact less adequate than the old view that "the phlogiston
theory crumbled under the cumulative force of Lavoisier’s evidence". An
improved version of this old view is what Kitcher tries to provide,
more successfully in my view than anyone else who has tried to do the
same. Kitcher is clearly aware of the various merits of the phlogiston
theory and, like various other well-informed commentators, grants that
there was initially no clear difference between the empirical adequacy
of the phlogiston theory and Lavoisier’s theory (ibid., p. 273).
However, Kitcher argues, the phlogiston theories were unable to deal
with the new empirical evidence that emerged in the 1780s.
As in many other arguments (starting with
Lavoisier’s own) designed to show the empirical inadequacy of the
phlogiston theory, Kitcher focuses on the weight relations in key
chemical reactions including combustion and calcination. Intuitively,
the main point is that in combustion/calcination nothing (such as
phlogiston) is emitted, but something (oxygen) is absorbed, as shown by
the fact that the reaction products, added together, weigh more than
the combustible substance (or the metal) before the reaction. Kitcher
avoids the common mistake of assuming that the phlogistonists simply
ignored the evidence, or that they fled into the idea of the negative
weight of phlogiston (which a small number of people did entertain).
Rather, he correctly notes (ibid., p. 277): "they do something
that is far more reasonable: to wit, accept Lavoisier’s claim that
something from the air is absorbed and try to combine this concession
with the traditional idea that phlogiston is emitted." But this
defensive strategy ran into dead-ends eventually, Kitcher argues. He
focuses on the work of Richard Kirwan, who tried to accommodate all
observed phenomena by postulating that a calx may contain water or
‘fixed air’ (our carbon dioxide), and that the combination of oxygen
and hydrogen can make water or fixed air depending on the temperature
(Kirwan 1789; Kitcher 1993, pp. 283-288). Kitcher is correct in noting
that Kirwan’s story ended in complex tangles, even inconsistencies with
some experimental results, such as the demonstration that no fixed air
could be extracted from calxes unless there were carbon impurities
present, regardless of the temperature to which they were subjected.
Thus Kitcher concludes that it was right that Kirwan himself accepted
defeat and gave up the phlogiston theory[3].
Kitcher contends that "the rest of the story is more of the same". But
that needs to be shown, not assumed. For example, Kitcher does not
assess the mature phlogiston theory advanced by Henry Cavendish (1784),
which was free of any contradictions or inordinate complexities as far
as I can see. Instead he discusses (ibid., p. 284) Cavendish’s
earlier view (1766), according to which inflammable air was pure
phlogiston, which was problematic considering that inflammable air
clearly had weight. As I will discuss further in Section 2.4, Cavendish
had a simple and straightforward view about what the combination of
hydrogen and oxygen would make (water, not fixed air); his view did not
have the sort of ambiguity that created trouble for Kirwan.
We can meaningfully engage in an in-depth
dispute
about just how much the empirical adequacy of the phlogiston theories
was compromised by various observations, especially regarding weight.
However, we need to do so without losing sight of a far more important
point: the relevant question of empirical adequacy is a comparative
one, not an absolute one. The question is not whether the phlogiston
theory was absolutely flawless (to which the answer is ‘of course
not’), but whether its empirical adequacy was better or worse than its
competitors at the time, particularly Lavoisier’s theory. We really
need to lose the habit of treating ‘phlogiston theory got X
wrong’ as the end of the story; we also need to ask whether Lavoisier’s
theory got X right, and whether it did not get Y and Z
wrong.
There has been a great tendency, among
philosophers
and historians alike, to ignore and minimize the things that
Lavoisier’s theory could not explain (or got wrong by modern standards)[4].
One might say that this is testimony to the effectiveness of
Lavoisier’s rhetorical offensive, which seems to have won over
generations of later commentators as well as his contemporaries.
Kitcher (1993, p. 278) is much more aware of this trap than most
detractors of phlogiston are, and he does note that there were
difficulties with Lavoisier’s theory of heat and his theory of acidity.
Yet Kitcher’s concession, that giving a verdict in favor of Lavoisier’s
theory "is not to say that his own analysis is free of problems", is
made briefly with no details, and then left behind[5].
And then he goes right back to an elaborate discussion highlighting
those issues on which the phlogiston theories had the most
difficulties. This biased emphasis, along with a near-dismissal of
Lavoisier’s problems, gives apparently strong support to Kitcher’s
assessment that by the mid-1780s Lavoisier had developed "a general
account which deals, in a unified and consistent way, with a far
greater range of the experimental results than any extant version of
the phlogiston theory." (Ibid., p. 278) At the end Kitcher (ibid.,
p. 289) does again acknowledge the need for a comparative viewpoint,
and briefly discusses how Lavoisier had to repel Kirwan’s attack
concerning Lavoisier’s table of affinities of oxygen.
A closer look at the primary literature
reveals
that there were a number of other observations and experiments known at
the time which Lavoisier’s theory failed to explain. First of all,
there was a profusion of curious and weird anomalous phenomena reported
by various phlogistonists that were simply brushed aside by Lavoisier
and his colleagues. To get a flavor of these phenomena, it would be
sufficient to have a casual glance through Priestley’s volumes on air
or Scheele’s collected papers. For example, Priestley reported on an
experiment in which he "impregnated" distilled water with "nitrous
vapour": "the water presently became warm, then began to sparkle very
much, air issuing from all parts of it very copiously; and after this
it assumed a light blue colour"; in a later run of the same experiment,
the water went on to become green, "about which time the emission of
air ceased; and lastly, after the green colour had deepened very much
[…] a yellowish tinge was perceived to be diffused through the green
colour." (Priestley 1790, pp. 336-8) Priestley continued to produce a
stream of diverse phenomena in the laboratory right up to his last
years. For example, he became excited about Volta’s invention of the
battery, and reported that he could not electrolyze water devoid of
dissolved air, and also that he had dissolved a gold wire in plain
water by using it as the anode in electrolysis (Priestley 1802).
Or look at Scheele’s 1774 paper on manganese,
which
is remembered now for the discovery of chlorine. What Scheele called
‘manganese’ was pyrolusite, or manganese dioxide (MnO2)
probably in an impure form. In reacting this mineral with muriatic acid
(or marine acid – hydrochloric acid, HCl, in modern terms) he produced
a poisonous yellow-green gas (chlorine), which he called
‘dephlogisticated muriatic acid’ because he thought it was the result
of the removal of phlogiston (hydrogen?) from the acid by the
manganese. He also went on to make a number of other observations. For
instance, Scheele (1931, pp. 24-25) reported that the reaction of
manganese with acids was facilitated by the presence of high-phlogiston
substances such as sugar, honey, gum arabic, or hartshorn jelly;
manganese filings dissolved only partially in acid alone, but dissolved
completely when combustibles like sugar were added to the acid[6].
Lavoisierians ignored all the complicated observations, and attempted
to contradict Scheele’s interpretation of chlorine by claiming that it
was ‘oxygenated muriatic acid’[7].
Even aside from such unruly phenomena there
were
other significant anomalies, which were recognized by Lavoisier himself
and his allies. When pressed, even the great Lavoisier-enthusiasts of
today will admit that he was quite mistaken in his theory of acids, in
which he proposed that oxygen was the essence of acidity. Apologists
tend to dismiss Lavoisier’s theory of acids as an incidental part of
his chemistry that can be safely discarded while preserving the other,
good parts. But Lavoisier was so enamored with his theory of acids that
he named his beloved oxygen to mean ‘acid-generator’. He was not
dissuaded by critics, including Cavendish (1784, p. 153), who pointed
out that muriatic acid and the ‘acid of tartar’ could not be deprived
of their acidity "by any union with phlogiston" (or, what came to the
same thing for Cavendish, by any attempt to extract oxygen from them).
While Lavoisier knew that there were certain acids that could not be
made to yield any oxygen, he was confident that improved techniques
would extract oxygen from them eventually. With such confidence he
included in his table of chemical elements the ‘muriatic radical’ (what
one would produce by removing oxygen from hydrochloric acid, HCl,
in modern terms), as well as the ‘fluoric radical’ and the ‘boracic
radical’.
In Lavoisier’s table of simple substances
(Figure
1) we also have a reminder of another problematic part of Lavoisier’s
theory, namely caloric, at the top of the table along with light.
Unlike the theory of acidity, ideas about caloric were undeniably
central to Lavoisier’s system, occupying the very first chapter of his
definitive textbook of the new chemistry (Lavoisier 1789). There are
many modern apologists, including Kitcher (1993, p. 278, footnote 70),
who try to downplay Lavoisier’s belief in the reality of caloric. They
ignore the key role that caloric played in his theoretical system, and
mistake as genuine and specific doubt what was merely common
lip-service to proto-positivistic caution about all theoretical entities[8].
The points I want to make here become the clearest in relation to
Lavoisier’s theory of combustion, which was indeed one of the most
important points of contention between Lavoisier and the
phlogistonists. And this is also where we encounter the most important
and most incredible anti-phlogiston prejudice in most modern
commentators.
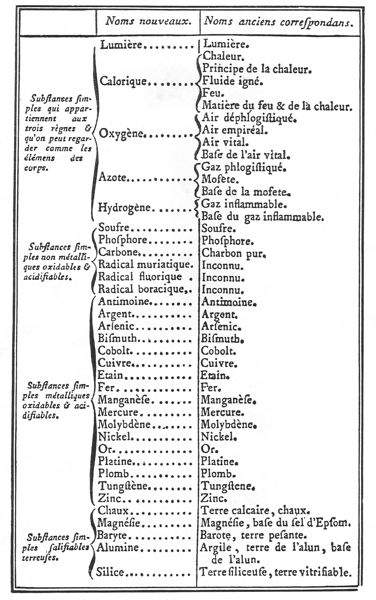
Figure 1: Lavoisier’s table of simple
substances (Lavoisier 1789, p. 192; p. 175 in the English translation).
Here we have to remember that Lavoisier
understood
combustion as involving a decomposition of oxygen gas into ‘oxygen
base’ and caloric, the oxygen base combining with the combustible, and
the caloric being released. The heat generated in combustion comes out
of the oxygen gas, and it is essential that the oxygen is in a gaseous
state to begin with, since it is the abundance of combined caloric
which puts matter into the gaseous state. The various difficulties of
this theory of combustion may not be discussed very often in histories
of the Chemical Revolution nowadays, but they were well known at the
time. Thomas Thomson (1802, vol. 1, pp. 354-8), for instance,
summarized them in his popular and authoritative textbook of chemistry.
The empirical anomalies of Lavoisier’s theory included cases of
combustion without involving oxygen in the gaseous state, and some
cases involving no oxygen at all. The phenomena simply did not follow
Lavoisier’s dictate that the production of heat was caused by the
liberation of caloric from a gas as it became condensed to a solid or
liquid state (and similarly with light); there were cases of heat
production when the reaction products were gaseous (e.g. the
burning of carbon), and cases in which gases were condensed by chemical
combination without much heat production. One high-profile difficulty,
discussed in illuminating detail by Seymour Mauskopf (1988), concerned
the combustion of gunpowder, no doubt prominent in Lavoisier’s own mind
as he worked from his laboratory at the Paris Arsenal. No less than
Claude-Louis Berthollet, later to be one of his most loyal allies,
challenged Lavoisier’s theory using this example: gunpowder combusted
very well in the absence of ambient oxygen gas; there was oxygen
contained in the gunpowder itself, but that was in the solid state.
All in all, the empirical adequacy of
Lavoisier’s
new chemical theory was highly questionable, and often questioned. It
is genuinely difficult to say whether Lavoisier’s theory was more or
less empirically inadequate than the phlogiston theory. This is by no
means a knock-down case of one theory being so clearly superior to
another that a careful consideration is not necessary. In order to give
a clear verdict, we would need an agreed-upon quantitative measure of
empirical adequacy that can give us a composite index from the variety
of phenomena that a theory covers more or less well. In the absence of
such an empirical adequacy measure, we may not be able to go beyond
Cavendish’s assessment at the time, which we would do well to remember,
at least (1784, p. 152): "as adding dephlogisticated air to a body
comes to the same thing as depriving it of its phlogiston and adding
water to it […] it will be very difficult to determine by experiment
which of these opinions is the truest; but as the commonly received
principle of phlogiston explains all phenomena, at least as well as Mr.
Lavoisier’s, I have adhered to that."[9]
(What exactly Cavendish meant here will be explained below.)
2.2 Kuhnian revolution
In many ways, Thomas Kuhn’s account of the
Chemical
Revolution is more informative than the basic empiricist line examined
above. References to the Chemical Revolution are scattered throughout The
Structure of Scientific Revolutions
and elsewhere in Kuhn’s work, but a convenient and insightful synthesis
can be found in a paper by Paul Hoyningen-Huene (2008), on which one
can rely at least for most purposes. Kuhn clearly recognizes the
difficulty involved in trying to say which side was better in the
Chemical Revolution. He notes the mismatch in the problem fields
handled well by the competing sides, and emphasizes that there were
different standards of judgment employed by them; these and other
paradigm-based differences clearly constitute an instance of
methodological incommensurability, although it is more debatable
whether there was any significant semantic incommensurability involved.
Kuhn in fact highlights Priestley’s resistance to Lavoisier’s new
paradigm as a case illustrating the lack of super-paradigmatic criteria
of rationality in science:
Though the historian can always find men –
Priestley, for instance – who were unreasonable to resist as long as
they did, he will not find a point at which resistance becomes
illogical or unscientific. At most he may wish to say that the man who
continues to resist after his whole profession has been converted has ipso
facto ceased to be a scientist. [Kuhn 1970, p. 159]
Ironically, these merits of Kuhn’s account of
the
Chemical Revolution also constitute its greatest defect, for those who
would seek philosophical explanations of scientific behavior. Kuhn says
that Priestley’s resistance was never irrational, and that it was only
unreasonable because he was being stubborn even after the great
majority of chemists had gone over to Lavoisier’s side. But why did the
majority of chemists change their minds, in the first place? It is well
known that a prominent group of sociologists of science took Kuhn’s
ideas to their logical conclusion (or, I should say, one of
their possible ‘logical’ conclusions), and declared that all scientific
decisions should be explained by reference to social factors (e.g.,
Barnes 1982).
If one (perhaps irrationally) wanted to
resist that
ascent to sociology, then it might seem that the only other way to go
is to fall back to the strategy of finding something, anything,
that is wrong with the losing side so that we can feel good about the
majority going with the winning side. An intriguing example of this
reactionary strategy can be seen in Howard Margolis’ book Paradigms
and Barriers.
Margolis’ ‘habits of thought’ is an interesting adaptation of Kuhn’s
ideas, especially referring to pragmatic roots of conceptual habits.
Margolis’s starting point is a puzzle: "the puzzle is to understand why
men as able as Priestley and Cavendish, and indeed Lavoisier himself,
found it so hard to give up the idea of phlogiston." (Margolis 1993, p.
43) Margolis wants an explanation as to why "even Lavoisier himself was
slow to make arguments against phlogiston", and why "when he did give
an argument that seems convincing today, no chemists followed." (p. 46)
This way of thinking is premised on the idea that Lavoisier’s theory
was really so much better than the phlogiston theory, and Margolis
secures that premise mostly by selective attention, for example
conveniently not mentioning caloric in Lavoisier’s explanation of
combustion (ibid., p. 44). Margolis’ answer to the puzzle is
that there was a habit of thought, based on the intuitive idea that in
combustion something (phlogiston) is emitted, that worked as a
cognitive obstacle to progress: the transition from phlogiston to
oxygen was "logically […] exhilarating", but "cognitively it was
plainly painful for most chemists" (ibid., p. 49). This type of
situation Margolis identifies as "a Kuhnian revolution: cognitively
difficult though logically not so, hence best understood as turning on
the presence of a barrier habit of mind." (Ibid., p. 50)
Margolis’ analysis is not exactly faithful to
Kuhnian ideas, nor is it intended to be. However, it does accentuate
some fundamental difficulties in broadly Kuhnian explanations of
revolutionary episodes. The Kuhnian framework naturally explains
agreement in normal science and disagreement in extraordinary science.
So, following Kuhn, we can easily explain why disputes between
competing paradigms can persist, but we have difficulty explaining why
and how those disputes do get resolved and end in agreement. This
difficulty is not felt in Margolis’ analysis, because he has no
compunctions about assuming that all chemists should have seen the
light and followed Lavoisier; then follows the semi-Kuhnian
explanation, that they would have done so, except for their attachment
to the ‘phlogiston escaping’ habit of thought. For those who follow
Kuhn more faithfully, it is not easy to be so cavalier about the
explanation of why those who ‘converted’ to Lavoisier did so. So we
come back to the general complaint about Kuhn’s treatment of
revolutions: it seems to provide no convincing reasons as to why a
scientist does or should go with one or the other of the competing
paradigms.
2.3 Simplicity and unity
Those disappointed by the lack of explanations
for
revolutionary change have tried to get beyond Kuhn in various ways. For
the moment, let me set aside the possibility that we really need
‘social’ explanatory factors, which I will come back to later. Kuhn
(1977) himself moved on to considering certain basic epistemic values
shared even by scientists in different paradigms. It is possible to
argue that when a revolutionary struggle in science does reach a
resolution in the triumph of one paradigm over another, that agreement
is generated because the winning paradigm is superior to the losing one
in terms of some of these super-paradigmatic values. Simplicity is one
epistemic value that has been invoked time and again in attempts to
explain the Chemical Revolution. On the surface, it is quite an
appealing notion that Lavoisier’s theory won because it was inherently
simpler than the phlogiston theory. The crudest version of this idea
says that the phlogiston theory unnecessarily complicated things by
postulating the existence of an unobservable substance, phlogiston. But
that is, again, to ignore the fact that Lavoisier had to postulate the
existence of an equally unobservable substance, caloric.
Perhaps the most sophisticated of these
simplicity-based arguments comes from Andrew Pyle (2000). The
sophistication of Pyle’s position is already evident in his handling of
the weight-gain issue: "the weight-gain phenomenon posed a genuine
difficulty [for the phlogistic chemists], and one which generated a
number of very different responses. It could not, however, be described
as a knock-down refutation." (Ibid., p. 109) In agreement with
Kitcher and Margolis, and with Alan Musgrave, whose ideas will be
discussed shortly, Pyle (ibid., p.
110) emphasizes that up to about 1783 Lavoisier’s theory had little
overall advantage. So it makes sense that few people converted up to
that point, and that Lavoisier himself did not launch an aggressive
campaign. All of this changed when Lavoisier arrived at the attractive
new hypothesis about the composition of water, namely that it was a
compound of hydrogen and oxygen, not an element as the phlogiston
theorists (and everyone else) had assumed. Unfortunately, Pyle’s
explanation of why most chemists did convert to the oxygen theory
quickly after 1783 is not satisfactory. One problem is that Pyle only
picks out rational-looking parts of the story. But even if we allow his
selection of events for the moment, his argument about their
rationality is very thin.
Pyle notes, quite rightly, that the
phlogiston
theorists had to concede that while the metals lost phlogiston in the
process of calcination, something else (such as water or fixed air)
became combined with the metal to give it extra weight. But why invent
and hold on to such complicated stories, when there was a simpler story
that did the job? Pyle also makes much of the fact that mainstream
phlogiston theory after 1783 was of a hybrid nature, that is,
acknowledging a clear chemical role for oxygen (by whatever name),
while maintaining the existence of phlogiston. And then, in the midst
of this highly nuanced discussion, Pyle suddenly descends into a
simple-minded point about simplicity:
By 1800, the old phlogiston theory was dead,
and the
outstanding dispute was between Lavoisier’s theory and a spectrum of
compromise-theories. How might such a debate be settled? Here the
factor of simplicity comes into play on the side of Lavoisier. His
theory of combustion is objectively simpler than compromise theories in
that it represents combustion in terms of 3 factors rather than 4.
[Pyle 2000, p. 113]
I take it that the three factors that Pyle
identifies in Lavoisier’s theory are: the combustible, oxygen base, and
caloric. On the phlogiston side, the factors involved must be all of
those, plus phlogiston. I am not sure why Pyle thinks that
phlogistonists necessarily needed caloric rather than using phlogiston
to account for heat (there were diverging opinions on this point among
phlogistonists), and why he is letting Lavoisier off the hook by
ignoring the fact that he also postulated the existence of lumière,
the substance of light, which was the very first item in his table of
simple substances (see Figure 1 above). Depending on how one counts,
the substance count could easily be four to three in favor of the
phlogistonists. In any case, it does not seem right to choose the
fundamental theory of chemistry on the basis of whether it postulates X
or X+1 substances. We would first need a good story about why
that kind of simplicity is so important.
Pyle (ibid., p. 114) also reinforces
a
slightly different simplicity-based argument in favor of Lavoisier,
which is more about the constancy and uniformity of opinion rather than
simplicity as such. Originally this was an argument that Lavoisier
himself made with much rhetorical effect: phlogiston was a ‘veritable
Proteus’, which changed its form just as needed, and no two
phlogistonists could agree about what it really was. At first glance it
does seem terrible that phlogistonists could not even agree amongst
themselves, while Lavoisier’s school had a unified stance. But on more
careful consideration this is not an argument that carries much weight.
It has no force when we are trying to consider the rationality of each
phlogistonist’s position. (Should Scientology rationally convince
Christians to give up Christianity because there are so many mutually
conflicting variants of the latter?) We also need to recognize that the
anti-phlogistic camp was not completely united, either. There was no
great and lasting unity among those who accepted oxygen and caloric
(for example, about whether light was a separate substance from
caloric, or about whether caloric was made up of particles). There were
also many other people who used neither caloric nor phlogiston,
preferring their own ideas about the nature of ‘elementary fire’ and
such. And there was considerable ontological discomfort and indecision
in general about the imponderables, on which not even all Lavoisierians
were in agreement. The Chemical Revolution was not a Manichean conflict
between the Lavoisierians and the phlogistonists. When all these facts
are taken into account, there is not much of substance left in the
arguments based on simplicity or unity for the rationality of the
Chemical Revolution.
2.4 Lakatos, Musgrave, and progress
In my view, the best available philosophical
treatment of the Chemical Revolution is still Alan Musgrave’s paper of
1976, which makes an application of Imre Lakatos’ methodology of
scientific research programs to this case. Musgrave argues that after a
certain point the phlogiston research program ceased to be progressive,
while the oxygen research program continued to be progressive.
Musgrave’s explanation is framed explicitly in Lakatosian terms, so
what he means by ‘progress’ is the production of successful novel
predictions, and the rational thing for scientists to do is to choose
the most progressive of available research programs. So the Chemical
Revolution is seen as a perfectly rational affair, and thereby also
vindicates Lakatos’ philosophy of science.
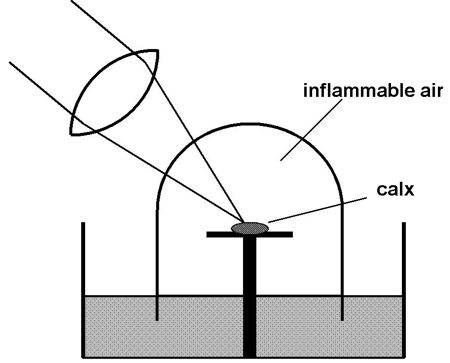
Figure 2. A schematic representation of
Priestley’s
experiment demonstrating the reduction of lead calx by heating in
inflammable air.
Musgrave (1976, p. 199) sets up the crucial
moment
of truth very nicely. The phlogiston program was highly progressive for
a time, up to Joseph Priestley’s prediction in 1783 that a metallic
calx (or, oxide) would be reduced (turned back into shiny metal)
through heating in inflammable air, which he considered to be
phlogiston itself at that time. This prediction received a stunning
corroboration in Priestley’s experiment of heating minium (lead calx)
in inflammable air by means of a large burning lens (see Figure 2).
Priestley declared: "I could not doubt but that the calx was actually
imbibing something from the air; and from its effects in making the
calx into metal, it could be no other than that to which chemists had
unanimously given the name of phlogiston." The moment of high
drama came when Lavoisier turned this apparent phlogistonist triumph
upside down by exploiting Cavendish’s new work on the production of
water by the combustion of inflammable air (ibid., p. 201).
Characteristically, Lavoisier began his counter-offensive by noting
that the lead calx in Priestley’s experiment would have lost some
weight in turning back into metal, as in other cases of reduction. Then
he deduced that the lost weight would have gone into the water that
must have been produced in the experiment, composed of oxygen coming
from the calx, and the ambient inflammable air (which he then re-named
‘hydrogen’).
Ironically, Musgrave points out, it was
Priestley
himself who confirmed Lavoisier’s prediction (or retrodiction) that
water must be (must have been) produced in the experiment, by
performing the experiment over mercury, instead of water as in the
original setup. But as Lakatos might have predicted from the general
nature of research programs, the phlogiston program was actually not
conclusively defeated at this point. Priestley, instead of converting
to Lavoisier’s theory at this point as Kitcher and Margolis would see
fit, switched to Cavendish’s new version of the phlogiston theory,
which hinged on the ingenious post hoc modification to the
effect that inflammable air was not phlogiston but phlogisticated
water, while oxygen (or, dephlogisticated air) was dephlogisticated
water. However, Musgrave argues (ibid., pp. 203-6), from this
point on the phlogiston theory was forever on its back foot, adjusting
itself this way and that way to accommodate inconvenient new findings
but not managing to make any successful novel predictions. As he puts
it (p. 203), "a degenerating programme can soldier on, and
phlogistonism did just that", using Cavendish’s new ideas. But at that
point the Lakatosian verdict kicks in: it is irrational to hold on to a
degenerating research program; it was rational for chemists to abandon
the phlogiston program after 1783 or so, and most chemists were indeed
rational in that way, leaving behind the ‘elderly hold-outs’ like
Priestley and Cavendish.
Musgrave’s argument certainly has some
plausibility, but there is a problem: where are the successful novel
predictions made by the oxygen program after the phlogiston program
stopped making them? Musgrave (ibid., p. 201) counts
Lavoisier’s
deduction that water must have formed in Priestley’s 1783 experiment as
a novel prediction. But this is a difficult claim to sustain.
Lavoisier’s analysis was only made in retrospect, though it can be said
that in the logical sense his theory ‘predicted’ the production of
water, which Priestley had failed to observe in the original
experiment. But, as Musgrave acknowledges, the same ‘prediction’ was
also made by Cavendish’s theory, and it was more likely the
phlogistonist Cavendish, not Lavoisier, who occasioned Priestley to
repeat the experiment over mercury. Lavoisier had not predicted the
production of water in the experiment of exploding hydrogen and oxygen
together, and his hypothesis about the composition of water was itself
a post hoc adjustment made in order to explain the unexpected
production of water in Cavendish’s experiment (exploding a mixture of
hydrogen and oxygen gases). According to Lavoisier’s original position,
the product of this reaction should have been an acid, since it
contained oxygen; Lavoisier tried in 1777 and 1781-82 repeatedly to
produce an acid by the combustion of inflammable air, without success
and without detecting the water produced in it, either. Musgrave (ibid.,
p.
199) tells us all of that, with perfect clarity. Lavoisier’s account of
the composition of water started its life not as a novel prediction,
but as a classic ad hoc hypothesis (lacking use-novelty as well
as temporal novelty).
Were there any successful novel predictions
made by Lavoisier? Musgrave (ibid., p.
203) gives us one: "water, traditionally used to put out fires, should,
since it contains oxygen, support slow combustion and yield hydrogen.
Iron filings immersed in water did indeed rust and hydrogen was
collected." But, again, this was just as deducible from Cavendish’s
1784 version of the phlogiston theory: if iron gave its phlogiston to
water, that would produce phlogisticated water, which is hydrogen. The
same can be said about Lavoisier’s famous decomposition of water vapor
by hot metal: the transfer of phlogiston from the metal to the water
would cause the former to turn into a calx, and turn the latter into
inflammable air (phlogisticated water). So these novel predictions do
not quite qualify as crucial experiments, and I cannot see any other
significant candidates for successful post-1783 novel predictions made
by the Lavoisierian research program.
Meanwhile, there were some distinctly
un-progressive aspects of the oxygen research program in the 1780s and
beyond, including some embarrassingly unsuccessful predictions, and
some unexpected new phenomena which Lavoisier and his followers could
only accommodate without the desired by-products of successful
novel predictions. As mentioned earlier, on the basis of his oxygen
theory of acids Lavoisier confidently predicted that muriatic acid (our
hydrochloric acid) would be decomposed into oxygen and the ‘muriatic
radical’. Lavoisierian responses to similar anomalies of prussic acid
(HCN, in modern terms) and sulphuretted hydrogen (H2S) also
had no progressive outcomes. In neutralizing Berthollet’s challenge
about the combustion of gunpowder, Lavoisier only managed ad hoc
hypotheses (in the Lakatosian sense of not resulting in successful
novel predictions)[10].
And Lavoisierians made pretty un-progressive responses to the discovery
that not only oxygen but also chlorine gas supported combustion, but no
other known gases did. So, if we stick to Lakatos’ criterion of
progressiveness, I think the verdict between phlogiston and oxygen is
actually quite ambiguous. In the end, Musgrave does not even give us a
convincing ‘rational reconstruction’ of the post-1783 phase of the
Revolution.
3. How we have got the explanandum wrong
The discussion in the preceding section can
be summarized with succinct pessimism: I am not aware of any
philosophical account that is sufficiently successful in explaining why
the vast majority of European chemists signed up to Lavoisier’s theory,
and I do not think it is likely that there will be a much better
account forthcoming. Faced with the kind of philosophical failure,
there are a few possible reactions. First, we could just keep trying
out new philosophical explanations; this would require a degree of
optimism verging on the desperate. Second, we could give up on
philosophical explanations altogether, and try for social explanations.
This is a tempting option, but it does not work out all that well for
the Chemical Revolution[11],
and I also have some general objections to the flight to the social,
which I will explain in Section 4. I would like to suggest a third
option, which is based on the suspicion that perhaps we are not finding
any good explanations because we are trying to explain something that
did not actually happen. (Imagine all the fun we could have trying to
explain, say, why Germany won the First World War despite the entry of
the U.S. into the war.)
If my suspicion is corroborated by
independent
historiographical work, then we will have made productive use of a
philosophical failure to improve historiography, as promised. The
history-philosophy interaction in this process will be the subject of
Section 5, but here let me just outline how it works out in the case of
the Chemical Revolution. My historical thesis, which I will attempt to
demonstrate in this section, is that the Chemical Revolution did not
consist in a swift and near-complete conversion of the chemical
community to Lavoisier’s theory. Here we need to resist being taken in
by triumphalist declarations of a clean victory originating from
Lavoisier himself, his contemporary advocates, and some posthumous
glorifiers of Lavoisier[12].
The assumptions of a clean victory can be found in some quite
unexpected places, too. For example, this is what Priestley himself
said, in the opening sentence of his latter-day defense of the
phlogiston theory issued from his exile in America in 1796:
There have been few, if any, revolutions in
science
so great, so sudden, and so general, as the prevalence of what is now
usually termed the new system of chemistry, or that of the Antiphlogistians,
over the doctrine of Stahl, which was at one time thought to have been
the greatest discovery that had ever been made in the science.
[Priestley 1796]
Maybe this was an exaggerated complaint from
the
loser, but strangely, the same idea can also be found in the works of
some very careful historians. For example, Robert Siegfried says:
Of all the well known revolutions in the
history of
science, the chemical is perhaps the most dramatic […]. Only twenty
years separate Lavoisier’s first explorations of the chemistry of gases
and the public capitulation of Richard Kirwan, the last significant
European defender of the phlogistic views. [Siegfried 1989, p. 31]
The impression of suddenness is shared by
Carleton Perrin:
Few of the major conceptual shifts in the
history of
science rival the chemical revolution for compactness in time and
consequent sense of drama. As usually defined, the episode spanned a
mere twenty years. [Perrin 1981, p. 40]
The impression of unanimity is voiced by Larry
Holmes (2000, 751): "all but Priestley himself eventually came over to
the side of the French chemists".
Now, it may well be that scientific
revolutions
usually take much longer than 20 years, so the Chemical Revolution was
a quick one in relative terms, but a reasonably close look at the
primary literature should make it evident that there were numerous
chemists who decided not to jump on the Lavoisier bandwagon even by
1790 and beyond, whom I will call ‘anti-anti-phlogistonists’. And this
has indeed been noted in various historical accounts, although to the
hapless philosopher looking for some historical work to draw from,
these scattered sources will not be easily visible. So, if nothing
else, the service I want to render here is to make a convenient and
useful summary of facts that are well-known to some experts here and
there. Many of the anti-anti-phlogistonists were respectable and
respected men of science, not just old men driven by sheer conservatism
or dogmatism. There were at least three different types of these
dissenters in the period after the publication of Lavoisier’s Elements
of Chemistry
in 1789, which is usually seen as the point at which the Chemical
Revolution was more or less complete, or at least irreversible (see
Table 1).
First of all, there were indeed some
die-hards.
Priestley tops this list, but he is only a small part of the picture.
One of the most striking figures is Jean-André De Luc, whose
objection
was based on his theory of rain, which postulated the transmutation of
atmospheric air into water[13].
De Luc also maintained close connections with various anti-Lavoisier
figures in Germany, particularly Göttingen, and also with
Priestley’s
associates in the Lunar Society of Birmingham, including James Watt. In
1796 Priestley identified the latter group as the only remaining
adherents to phlogiston that he knew of, in addition to Adair Crawford,
who had just died (Priestley [1796] 1969, p. 20). On the German side,
Karl Hufbauer (1982, pp. 140-4) notes that most chemists there either
converted to the Lavoisierian side or at least gave up any active
resistance by 1796, but allows that there were some remaining
phlogistonists, including Johann Christian Wiegleb and Johann Friedrich
Westrumb, who were ‘virtually ostracized’. And then there were people
like Torbern Bergman in Sweden and James Hutton in Scotland, whose
concerns were mineralogical and geological above all else. Hutton, for
example, had a notion of the circulation of phlogiston in the
environment which smacks of modern ecology’s understanding of the
cycles of carbon and energy, according to Douglas Allchin (1994).
Scheele did not survive long enough to prove his ‘die-hard’
credentials, but up to his death in 1786 he showed no sign of
relinquishing the phlogiston theory. Even right there in Paris there
remained significant anti-Lavoisierian figures, including Jean-Claude
Delamétherie, the editor of the prestigious Journal de
physique (called Observations sur la physique before 1794),
who followed Priestley’s ideas and cultivated a connection with De Luc[14].
There was also Jean-Baptiste Lamarck, whose idiosyncratic chemical
ideas are understood by Leslie Burlingame (1981) as belonging to the
natural-historical tradition of French science. To the list of French
die-hards, Perrin (1981, p. 62) also adds Antoine Baumé and
Balthazar-Georges Sage.
Table 1. Varieties of anti-anti-phlogistians,
in the order of birth in each category
|
Die-hards (‘elderly holdout’,
some not so
elderly)
|
Fence-sitters
|
New anti-Lavoisierians
|
|
James Hutton (1726-1797)
Jean-André De Luc (1727-1817)
Johann Christian Wiegleb (1732-1800)
Joseph Priestley (1733-1804)
Torbern Bergman (1735-1784)
James Watt (1736-1819)
Carl Wilhelm Scheele (1742-1786)
Jean-Claude Delamétherie
(1743-1817)
Jean-Baptiste Lamarck (1744-1829)
Adair Crawford (1748-1795)
Johann Friedrich Westrumb (1751-1819)
|
Pierre-Joseph Macquer (1718-1784)
Henry Cavendish (1731-1810)
Georg-Christoph Lichtenberg (1742-1799)
Lorenz Crell (1745-1816)
Claude-Louis Berthollet (1748-1822)
Johan Gadolin (1760-1852)
Friedrich Gren (1760-1798)
Jeremias Richter (1762-1801)
|
Count Rumford (1753-1814)
George Smith Gibbes (1771-1851)
Thomas Thomson (1773-1852)
Johann Wilhelm Ritter (1776-1810)
Humphry Davy (1778-1829)
|
The second category of dissenters sought
compromise, or deliberate neutrality. Allchin (1992), in his aptly
titled paper ‘Phlogiston After Oxygen’, makes a persuasive case that
many chemists admitted the existence of oxygen for gravimetric
considerations, while keeping phlogiston for what we would call energy
considerations. J.R. Partington and Douglas McKie, in their series of
papers on the phlogiston theory (1937-39, pp. 125-7, 143-8), already
pointed to a large number of people in this category, many of them
German or German-speaking, including Friedrich Gren, Lorenz Crell,
Jeremias Richter and Johan Gadolin. Hufbauer’s study (1982) of the
German chemical community in the 18th century has elaborated further on
that point. More generally, people often accepted Lavoisier’s theory
only partially, picking and choosing what made sense to them. The old
phlogistonist P.J. Macquer was taking this kind of approach when he
died in 1784, and even Lavoisier’s close colleague and ally
Claude-Louis Berthollet remained skeptical about some of Lavoisier’s
ideas, especially his theory of acids[15].
There were many others who clearly saw some merit in Lavoisier’s
chemistry but did not consider the evidence sufficient to reach a clear
verdict in favor of it. As discussed above, Cavendish (1784, pp. 150-3)
gave a clear-headed view of how both theories could explain the
phenomena he observed, while expressing a preference for staying with
phlogiston. Alfred Nordmann (1986) explains how Georg Christoph
Lichtenberg made a strong case that there was not enough knowledge yet
for a decisive verdict, and how annoyed he was by the Lavoisier group’s
attempt to legislate the language of chemistry, by which act they
forced other people to make a premature choice.
Even more interesting is the third category
of
dissidents, who fully acknowledged that Lavoisier’s system had become
established but also sensed that its time was passing quickly. Very
suggestive in this connection is the following snippet of scientific
conversation that I happened to stumble upon recently, from the year
1800. William Herschel had just detected infrared radiation coming from
the sun, which he saw as caloric rays separated from light rays by
means of the prism. Joseph Banks wrote to congratulate Herschel on this
momentous discovery, but had one piece of advice:
I think all my friends are of the opinion that
the
French system of Chemistry, on which the names lately adopted by their
Chemists are founded, already totters on its base and is likely soon to
be subverted. I venture therefore to suggest to you whether it will not
be better for you […] to use the term Radiant Heat instead of Caloric;
by the use of which latter word it should seem as if you had adopted a
system of Chemistry which you have probably never examined. [Banks to
Herschel, 24 March 1800, quoted in Lubbock 1933, 266-7]
Herschel accepted Banks’s advice happily: "I
have
the honour of your letter and shall be very ready to change the word
caloric for radiant heat, which expresses my meaning extremely well."[16]
Banks was a botanist and not a well-known chemist, but if the longtime
President of the Royal Society and ‘all his friends’ were predicting
the imminent demise of the French chemistry in 1800, then there must be
something that we have missed out in our usual historiography.
What did Banks have in mind when he said that
the
French chemistry was "tottering on its base"? It is impossible to say
for sure, but there are some clear things he might have had in mind. In
Section 2.1 above I have explained the empirical difficulties with
Lavoisier’s theory of acidity and with his theory of combustion. Thomas
Thomson (1802, p. 358), whom I already cited there, concluded: "upon
the whole, it cannot be denied that Lavoisier’s theory does not afford
a sufficient explanation of combustion." Thomson was not advocating a
return to phlogiston, but he wanted chemistry to move on beyond
Lavoisier. There was also growing discontent with Lavoisier’s caloric
theory of heat in general – particularly in London, where around 1800
there was a remarkable concentration of advocates of the notion that
heat was a form of motion, including Count Rumford, Humphry Davy,
Thomas Young, and Henry Cavendish.
The year 1800 is also significant because it
saw
the invention of the battery (or the ‘pile’) by Alessandro Volta. The
news reached England in the form of a long letter from Volta to Banks,
who had it printed in the Philosophical Transactions of the Royal
Society.
While waiting for its publication Banks showed Volta’s letter to his
friend Anthony Carlisle, a London-based physician. Carlisle repeated
Volta’s experiments with the help of the scientific publisher William
Nicholson, and they also used Volta’s pile to effect the first
decomposition of water using an electric current. This result was
reported in Nicholson’s own Journal of Natural Philosophy,
Chemistry and the Arts,
and caused quite a sensation. Now, the electrolysis of water into
hydrogen and oxygen would seem like great news for the Lavoisierians:
what could be a clearer proof of Lavoisier’s hypothesis about the
composition of water, now obtained without any complications involving
the calcination of metals and such things? Nicholson and Carlisle would
have agreed, but they added a puzzled note:
We had been led […] to expect a decomposition
of the
water; but it was with no little surprise that we found the hydrogen
extricated at the contact with one wire, while the oxygen fixed itself
in combination with the other wire at the distance of almost two
inches. This new fact still remains to be explained, and seems to point
at some general law of the agency of electricity in chemical
operations. [Nicholson 1800, p. 183]
This problem was noted by many others, and in
the
hands of young Johann Wilhelm Ritter in Germany it became a great
weapon against Lavoisierian chemistry. Ritter carried out various
experiments in support of his idea that electrolysis was not
decomposition at all, but a pair of synthetic reactions: negative
electricity comes in at one end and combines with water, and the
product of that combination is hydrogen; likewise, positive electricity
combines with water at the other end, and makes oxygen. According to
Ritter, water was an element after all, and hydrogen and oxygen were
water-based compounds. If you think of negative electricity as
phlogiston, Ritter’s view on water maps very neatly onto Cavendish’s
earlier view that hydrogen was phlogisticated water and oxygen was
dephlogisticated water. And there had indeed been many chemists who
suspected a deep connection between phlogiston and electricity before
this, as W.M. Sudduth (1978) records in a sadly neglected paper[17].
Ritter was the darling of the German Romanticists, and it seems that
his view had some advocates abroad, too. Again, we can see Lavoisier’s
system tottering on its base, as a consequence of new developments in
which Banks and his friends had a hand[18].
Perhaps the most interesting case of the new
generation of anti-Lavoisier chemists was Humphry Davy, who was still a
boy of about 10 years when Lavoisier’s Elements of Chemistry
was published. Davy later objected to almost every major aspect of
Lavoisier’s chemistry. He made his name in electrochemistry, and also
by putting a nail in the coffin of Lavoisier’s theory of acids with his
argument that chlorine was an element and muriatic acid did not contain
oxygen, only hydrogen and chlorine[19].
After the acceptance of Davy’s work, Lavoisier’s oxygen theory of
acidity was clearly dead, never to be revived again. As mentioned
above, Davy was also one of those who mounted serious challenges to the
Lavoisierian caloric theory of heat, whose dominance was never total[20].
As Siegfried (1964) reports in some detail, Davy actually entertained
various systems of chemistry involving the revival of phlogiston. David
Knight remarks (1978, p. 4): "there were widespread hopes and fears
until at least 1810 that Davy would restore it [the phlogiston theory]
and overthrow the French doctrines." Among those who expressed such
hope in print was George Smith Gibbes, doctor and chemical lecturer in
Bath, later to be physician to Queen Charlotte; in 1809 Gibbes opined
that Davy’s discoveries had confirmed that Lavoisier was wrong after all[21].
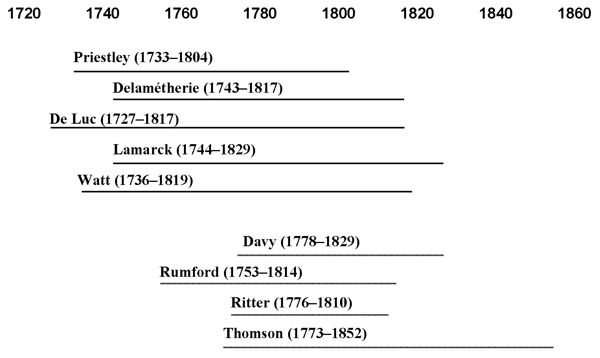
Figure 3. The overlap between old (top) and
new anti-anti-phlogistonists (bottom).
What can we say after all of that, about what
the
Chemical Revolution really consisted in? We still have to admit that a
considerable number of chemists became fully ‘converted’ to Lavoisier’s
chemistry at least for a time, and that it achieved a clear dominance
in the textbooks. However, we also need to acknowledge that there were
common cases of partial or half-hearted converts, and many of those
retained phlogiston in their systems. Add to that not only the die-hard
phlogistonists, but also the younger generation of dissidents who
actually had their scientific education after Lavoisier’s victory. A
very interesting thing about these two generations is that they in fact
overlapped significantly in time, the new generation coming up before
all the die-hards had given up (see Figure 3). Knight (1978, p. 29)
actually understates the case when he says, in reference to a later
episode: "As had happened with gothic architecture, this phlogiston
survival was almost contemporaneous with the phlogiston revival"[22].
There are many senses in which there was a ‘revolution’ in chemistry
effected by Lavoisier and his colleagues, but it was not a sudden and
clear-cut affair. It was a many-sided struggle that neither ended in
unanimous agreement nor established a long-lasting orthodoxy[23].
4. How do we explain what did happen?
With the new description of the Chemical
Revolution sketched in the preceding section, we can find a good
philosophical explanation of why it happened. The full story is too
complex to fit into this paper, as the Chemical Revolution was a
complex and multi-faceted event[24].
But one key point is simple: since Lavoisier had some excellent
arguments but lacked knock-down punches, it makes perfect sense that
some people shifted their allegiance to him, and others did not. To
borrow Kuhn’s phrase (1970, p. 94), since the dispute could not be
"unequivocally settled by logic and experiment alone", it makes sense
that there would have been continuing attachment to phlogiston in
various quarters. Having noted that there were many who were not sold
on Lavoisier’s chemistry, we no longer have to agonize about explaining
why the vast majority of chemists converted to Lavoisier’s chemistry.
Many did not, and that is quite easy to explain as a rational epistemic
response to the situation as it was. At the surface level, that is all
we need to say by way of a philosophical explanation of the Chemical
Revolution.
What is somewhat more challenging to explain
is why
those people who went over to Lavoisier’s side did so. So the original
difficulty I started with comes back to us in a revised form: what we
are seeking now is not an explanation of unanimity, but reasons that
impressed particular individuals and particular groups. Large-scale
social and political factors do not help the explanation in this case,
as the acceptance or rejection of Lavoisier’s theory easily cut across
lines of nationality, age, political ideology, economic and social
class, or profession. Instead, we would need to consider case-by-case
the interaction of various background factors that would have
influenced different individuals differently. Understanding individual
cases will require in-depth studies of the individuals concerned, and
that is beyond the scope of this paper. But there are some general
factors that would have affected a good number of people.
I do not share the methodological commitment
shown
by some social and cultural historians, that all explanations in the
history of science must be social. I am more inclined to seek any and
all explanatory factors that will, together, deliver a good
explanation. It is possible that the only correct explanations of the
Chemical Revolution are social or ‘external’ ones, but we cannot be
certain of that until we have also considered the scientific,
philosophical, or ‘internal’ explanations and assessed their relative
importance. In the spirit of considering all potentially relevant
factors, let me highlight two of them, while I do not pretend to be
comprehensive[25].
First, Lavoisier and his colleagues did run
an
effective and well-coordinated campaign for their new chemistry,
including the spreading of their new nomenclature and the controlling
of institutional spaces such as the Paris Académie and
the new journal Annales de chimie.
They also co-opted many of phlogistonist successes and reforms,
creating an exaggerated sense of revolution where there was in fact a
good deal of continuity; J.B. Gough (1988, p. 15) argues that
"Lavoisier owed a great deal more to his French Stahlian predecessors
than he was willing to admit publicly", and John McEvoy (1988; 2010)
has made a thorough assessment showing that Lavoisier’s chemistry was
not such a simple, abrupt departure from the chemistry that preceded
it. Mi Gyung Kim’s (2003, p. 390) observation is very apt: "the most
enduring elements of the Revolution, such as the analytic definition of
elements and the nomenclature reform, were not his."[26]
Lavoisier also had the ability for a very clear and systematic
exposition of ideas, which leading phlogistonists such as Kirwan and
Priestley lacked. But it seems to me that these factors are not quite
sufficient for explaining many of the conversions; they are all devices
that could have (and were) resisted by those who had other reasons to
oppose Lavoisier’s theory. For instance, the new nomenclature only
reinforced the habits of the already converted and indoctrinated the
younger generation who had to grow up learning chemistry in its terms.
As mentioned above, mature chemists who were not inclined to adopt
Lavoisier’s system were only irritated by the new nomenclature; they
saw right through it, as an attempt by the Lavoisier gang to impose
their theory on everyone before arguments had been considered carefully
enough.
Secondly, the rejection of phlogiston makes
much
more sense when we see it as a ripple riding on a large wave, which was
the very gradual establishment of the building-block ontology of
chemical composition. This point has been considered important only by
a small number of historians and almost no philosophers[27].
The phlogiston theory was grounded in the old chemical notion of
‘principles’, that is to say, basic substances which actively modified
other substances and imparted certain characteristic properties to them
– for instance, phlogiston was a principle which imparted
combustibility or metallic properties to substances it combined with.
This principlist[28]
thinking did not fit well with the building-block ontology, in which
all pieces of matter had equal ontological status (and the conservation
of weight before and after a reaction was a major concern). It is not
that the building-block ontology was entirely absent from the
phlogiston theories. It was present, but in a very uncomfortable mix
with principlist ontology. So we can actually imagine the metaphysical
relief in being able to do chemistry entirely on the building-block
basis. In fact Gough goes as far as to argue that "Lavoisier did not
initiative a revolution in chemistry: rather, he seized hold of a
revolution already in progress – a revolution that concerned the
composition of the chemical molecule – and tacked his own colors on to
it"; that revolution already in progress had been launched by the
French Stahlians, within whose compositionist system the phlogiston
theory created internal contradictions (Gough 1988, pp. 15, 29).
Phlogiston was washed out in the tide of the weight-focused chemistry
of the grouping and re-grouping of stable component units.
It is important for historians of this period
to
see beyond the clash between phlogiston and oxygen. If we want to
conceive of the Chemical Revolution as the event that gave rise to
‘modern chemistry’, we must follow Siegfried and Betty Jo Dobbs (1968)
in concluding that the endpoint of the Chemical Revolution was not
Lavoisier, but Dalton. What we are talking about here is not the actual
John Dalton immersed in the physics of caloric, but Dalton as sanitized
by later atomists, simply focused on immutable atoms as chemical units
with definite weights which worked as simple building blocks of the
chemical universe. In this ontological revolution, which I characterize
as ‘compositionist’, Lavoisier was actually not a thoroughly modern
figure since he was still partly steeped in principlist thinking, as
various historians have pointed out. William H. Brock (1992, pp.
112-3), for example, notes the irony that Lavoisier’s oxygen theory of
acidity was a direct descendant of none other than Stahl’s idea that
vitriolic acid was the ‘universal acid’, or the principle of acidity.
One could also argue that Lavoisier’s caloric was another principle,
which imparted the property of fluidity and elasticity to matter.
Perrin (1973, pp. 97-101) takes this point further, and argues quite
persuasively that the first five simple substances in Lavoisier’s table
were all principles (lumière, calorique, oxygène,
azote (nitrogen) and hydrogène;
see Figure 1 above). Lavoisier’s willingness to allow imponderable
substances in his system was also not fully harmonious with his own
emphasis on weight as the most important chemical property to keep
track of.
The ‘oxygen theory’ as crafted by Lavoisier
was a
rather fragile thing, whose impressive dominance cannot be explained
without reference to scientific fashions in the end. However, it is
also important not to be carried away with this observation. If the
demise of phlogiston had been simply due to the Lavoisier fad, then
phlogiston would have returned after the Revolutionary execution of
Lavoisier in 1794 and the slow dissolution of the well-disciplined band
of French scientists around him. But phlogiston never did return in
great force, and most of the new anti-anti-phlogistonists discussed
above were not phlogistonists. It may have been Lavoisier and his
friends who killed phlogiston, but there was a greater force at work
which kept it dead. Post-Lavoisierian chemistry was
resolutely compositionist, and that is what prevented the return of
phlogiston even after the dismantling of some of Lavoisier’s
fundamental ideas.
5. The interplay between history and philosophy
I would like to close with some reflections
on how history and philosophy interact with each other in the kind of
work I have showcased in this paper. The interactive process can be
schematized as follows. We start with facts about the past as given by
existing historiography. In trying to explain those facts
philosophically, we may fail. We may use that failure as a stick to
beat philosophers with, but we may also use it as an occasion to
re-examine the history. It is easily possible that we would decide that
the historiography we started out with was defective and needs to be
upgraded. (If so, we can come back to the philosophical task and see if
we can provide a good explanation of the updated history.) This is what
I meant about how philosophical failure can generate historiographical
refinement. I think this is one important mode of productive
interaction between history of science and philosophy of science.
It is my belief that there are also many more
modes
of history-philosophy interaction waiting to be articulated more
clearly and practiced more widely. For example, here is a model of how
we can use a failure of understanding at the history-philosophy
juncture in order to improve philosophy (rather than history), which
has been very important in my own work so far[29].
We start with existing philosophical frameworks, and find
historiographical puzzles, namely episodes that are difficult to
describe, and understand. In attempts to find an apposite description
of these episodes, philosophers can generate new concepts and ways of
thinking that they may not be led to otherwise. This is not so
different from the Lakatosian use of history as an evidence-base for
philosophy of science, which is taken to provide historiographical
research programs (Lakatos 1976).
Now, returning to the mode of the
history-philosophy interaction that I am focusing on in this paper,
historians may object that philosophical failure is not necessary for
the improvement of historiography, and that history can proceed and
refine itself on its own. That is logically true, but in practice
historians left to their own devices are not likely to have the
particular type of focus that the concern with philosophical
explanations generates, or reach the same kind of synthesis even when
the same historical facts are discovered. For me it has been a very
interesting experience to use my philosophical lines of inquiry to
discover not only neglected historical facts in the primary sources,
but also to unearth some sadly neglected secondary sources. This kind
of heuristic function for the improvement of historiography is not
restricted to philosophy; it can be performed by any field that
provides an explanatory framework for historical events and trends –
sociology, psychology, or economics, for instance.
Coming back to philosophy, we need to ask:
what is
philosophical understanding, and is it a kind of thing historians can
appreciate and even participate in? I want to argue that philosophical
understanding is based on intellectual empathy, which the historian of
science also cannot do without. This is perhaps a broader sense of
philosophical understanding than people normally have in mind, so let
me expand on it a little bit.
The initial argument regarding the Chemical
Revolution that I made was that we had not yet made good sense of the
18th-century chemists’ decisions. Then I argued that the basic problem
in that situation was a mistaken notion of what those decisions in fact
were. But there is also a common error in the philosophical discourse,
namely the assumption that making sense of past scientists’ decisions
must mean fitting them into our present conception of scientific
rationality; Lakatos is emblematic here, but he is by no means alone.
It is understandable that historians of science tend to have a violent
reaction against this mistake, but I also think that historians have
tended to throw the baby out with the bathwater in that reaction. It is
not necessary, or advisable, to shun all philosophical understanding as
a way of avoiding the imposition of particular modern conceptions of
rationality[30].
What the philosophical understanding of past
science requires is a broader and less restrictive sense of
intellectual empathy, a sense that we can see why past scientists would
have had the thoughts and beliefs that they had. Such intellectual
empathy is both an aim and a presumption in the business of
philosophical understanding, which philosophers should be very familiar
with from their work in the history of philosophy. And that is not so
different from the kind of understanding that anthropologists try to
reach about alien cultures, and historians try to reach about the past.
A common mistake in recent historiography of science is to imagine that
intellectual empathy can be reached by effacing our own selves
entirely. Kuhn used to say that the task of the historian of science in
studying a past scientist was ‘to get into his head’. (That reminds me
of the wonderfully quirky movie called Being John Malkovich,
whose protagonists discover a rabbit-hole on floor 7 1/2 of a
certain
office building that lands them inside John Malkovich’s mind; one
enjoys the privilege of being Malkovich for a little while, and then
gets dumped on the side of the New Jersey Turnpike; a return visit is
irresistible.) Once we get into Joseph Priestley’s head, how do we
navigate our way in there? Ultimately, I have to make sense of
Priestley’s thinking in my own way. Unless we can actually be brought
up in the community of past scientists (which would require another
science fiction movie), we are going to have to bring in some
conceptual framework from our own lives as we try to understand the
past scientists. The ideal of only using actor’s categories is an
impossible aim, and it can become pernicious if it is used as a blunt
weapon against any attempt to reach a kind of historical understanding
that accommodates the historian’s inevitable rootedness in the present.
The Chemical Revolution has been important
both as
an inspiration and as an illustration of the particular mode of
history-philosophy interaction that I have described in this paper. It
has been a particularly challenging case for philosophical explanation,
and this kind of challenge represents a major reason why historians of
science became disenchanted with the philosophy of science. But in the
case of the Chemical Revolution the philosophers have had an understandable
difficulty, faced with an impossible thing to explain; in this case the
historians have been as much to blame as the philosophers for creating
and maintaining misguided accounts of events.
In such situations philosophers would be
right to
send the case back to the historians as it were, or to engage in some
do-it-yourself history if the historians are not amenable. As long as
we do not insist in an overly narrow or particular kind of rationality,
the demand for rationality and the search for empathetic understanding
can be useful historiographical tools. The demand for understanding can
be a most effective type of challenge to misguided or distorted
descriptions, leading to corrections and supplements. Therefore,
philosophical pigheadedness in the face of failure of understanding can
serve as an effective method of historical discovery. In that mode of
critical scholarship bringing history of science and philosophy of
science together, all we are ultimately doing is to insist: "This
doesn’t make sense – we must go back and check if the story is correct."
Acknowledgments
This paper is based on a presentation that I
gave at the conference "Do Historians and Philosophers of Science Have
Anything to Say to Each Other?" at Duke University on 24 March 2007. I
would like to thank Seymour Mauskopf and Tad Schmaltz for their
encouragement, and various other participants for their stimulating
reactions. I thank Mimi (Mi Gyung) Kim for inviting me to that
conference, and for recommending this paper to Hyle. I also
thank two anonymous referees for very helpful comments.
Notes
[1] What is
contained in this paper is an elaboration of one part of the first
chapter of my book in progress, Is Water H2O? Evidence,
Realism and Pluralism (forthcoming from Springer).
[2] For an
informative discussion along similar lines, see Musgrave 1976, pp.
182-6.
[3] For more
detailed accounts of the development and downfall of Kirwan’s theory,
see Mauskopf 2002 and Boantza 2008.
[4] For further
details on that point, see Chang 2009, section 2.
[5] Kitcher also
adds an
apologetic footnote quoting cautious statements from Lavoisier showing
that he had less than an absolute commitment to his theories of heat
and acidity. But all that these statements show is that Lavoisier knew
how to distinguish theories from facts; that does not make the
ill-fitting facts any less disconfirming of the theory.
[6] Scheele
reasoned that
manganese required additional phlogiston to enable its reaction with
acid; when there was no external source of phlogiston, the outer parts
of manganese filings had to take the necessary phlogiston from the
inner parts, rendering the latter unreactable.
[7] For further
details on chlorine, phlogiston, and muriatic acid, see Chang and
Jackson 2007, chapters 1 and 2.
[8] For a detailed
discussion of
the real and beneficial role played by caloric in the chemistry and
physics of this time, see Morris 1972 and Chang 2003.
[9] After making
this statement,
Cavendish gave one marginal reason (other than conservatism) that
inclined him to favor phlogiston, which had to do with the composition
of plant materials.
[10] For details
on the debate regarding the combustion of gunpowder, see Mauskopf 1988.
[11] A brief yet
useful rebuttal
of some standard ‘external’ explanations is given by Musgrave 1976, pp.
206-7; for a fuller account, see Chang forthcoming, chapter 1.
[12] For critical
reviews of posthumous myth-making on Lavoisier, see Bensaude-Vincent
1983, 1996 and Kim 2005.
[13] Middleton
1965, pp. 115-131
gives a discussion of De Luc’s theory of rain. See De Luc 1803, pp.
1-306, for his detailed objections to the new chemistry, first in
itself and then in relation to meteorology.
[14] On
Delamétherie’s opposition to Lavoisier, see Guerlac 1975, pp.
105-6.
[15] On Macquer,
see Holmes 2000, p. 752; on Berthollet, see Le Grand 1975.
[16] Herschel to
Banks, 26 March 1800, quoted in Lubbock 1933, pp. 266-7.
[17] John Elliott
in 1780 even
proposed that phlogiston should be re-named as ‘electron’. See Chang
2009, Section 3, for more on this phlogiston-electricity connection.
[18] See Wetzels
1990 on Ritter’s
life and work in general, and pp. 208-9 on his interpretation of the
electrolysis of water. For a full discussion of the early history of
the electrolysis of water, see Chang forthcoming, chapter 2.
[19] See Golinski
1992, chapter 7. On chlorine, see also Chang and Jackson 2007, chapter
2.
[20] By the time
the energy
concept and early thermodynamics toppled the caloric theory altogether
in the 1840s and the 1850s, Lavoisier’s basic picture of the universe
was in tatters; later the kinetic theory would fill in the theoretical
vacuum in regard to the explanation of the three states of matter.
[21] See Golinski
1992, p. 213, who calls Gibbes "perverse" for this.
[22] Knight here
refers to
Stevenson 1849 and Odling 1871; for a full discussion of William
Odling’s ‘revival’ of phlogiston, see Chang 2009, section 3.
[23] After
outlining the
strategies and factors that led to the "triumph of the
antiphlogistians", Perrin 1981, pp. 61-63 ends by acknowledging the
complexity of the field: "Throughout this essay I have spoken of two
factions, one phlogistic and the other antiphlogistic. This, of course,
is an oversimplification. […] There was a wide range of attitudes
toward the claims of the new theory ranging from highly sympathetic to
hostile."
[24] I make my
best attempt at a full story in Chang forthcoming, chapter 1.
[25] McEvoy 2010,
chapter 6 gives an informative survey of various contextual accounts of
the Chemical Revolution.
[26] She
continues: "The oxygen
theory of acids and the caloric theory of heat, which could be regarded
as his unique contributions, quickly fell into disrepute." (Kim 2003,
p. 390)
[27] Siegfried
(1982, 2002) has
perhaps done more than anyone to initiate this angle on the Chemical
Revolution, which is reinforced by Klein & Lefèvre’s 2007
history
of materials in 18th-century chemistry. Also see Klein 1994, 1996 on
the origin of the concept of chemical compound, Multhauf 1966, 1996 on
the industry-concept connections, and Holmes 1971 and Debus 1967 on the
history of methods in analytical chemistry.
[28] Some
secondary sources use
the term ‘principalist’ in this connection, but I do not see the need
to modify the spelling in that way, which will only invite a false
association with the word ‘principal’.
[29] For example,
much of Chang 2004 consists of research in this vein.
[30] For a
further discussion of whiggism and historical judgment, see Chang 2009.
References
Allchin, D.: 1992, ‘Phlogiston After Oxygen’, Ambix,
39, 110-6.
Allchin, D.: 1994. ‘James Hutton and
Phlogiston’, Annals of Science, 51, 615-35.
Barnes, B.: 1982, T. S. Kuhn and Social
Science, New York: Columbia University Press.
Bensaude-Vincent, B.: 1983, ‘A Founder Myth in
the
History of Science? – The Lavoisier case", in: L. Graham, W. Lepenies
& P. Weingart (eds.), Function and Uses of Disciplinary
Histories, Dordrecht: Reidel, pp. 53-78.
Bensaude-Vincent, B.: 1996, ‘Between History
and Memory: Centennial and Bicentennial Images of Lavoisier’, Isis,
87, 481-99.
Beretta, M. (ed.): 2005, Lavoisier in
Perspective, Munich: Deutsches Museum.
Boantza, V.: 2008, ‘The Phlogistic Role of
Heat in
the Chemical Revolution and the Origins of Kirwan’s ‘Ingenious
Modifications … Into the Theory of Phlogiston’, Annals of Science,
65, 309-38.
Brock, W.H.: 1992, The Fontana History of
Chemistry, London: Fontana Press.
Burlingame, L.J.: 1981, ‘Lamarck’s Chemistry:
The Chemical Revolution Rejected’, in: H. Woolf (ed.), The Analytic
Spirit: Essays in the History of Science in Honor of Henry Guerlac,
Ithaca and London: Cornell University Press, pp. 64-81.
Cavendish, H.: 1784, ‘Experiments on Air’, Philosophical
Transactions of the Royal Society, 74, 119-53.
Chang, H.: 2003, ‘Preservative Realism and Its
Discontents: Revisiting Caloric’, Philosophy of Science, 70,
902-12.
Chang, H.: 2004, Inventing Temperature:
Measurement and Scientific Progress, New York: Oxford University
Press.
Chang, H.: 2009, ‘We Have Never Been Whiggish
(About Phlogiston)’, Centaurus, 51, 239-64.
Chang, H.: forthcoming, Is Water H2O?
Evidence, Realism and Pluralism, Dordrecht: Springer.
Chang, H. & Jackson, C. (eds.): 2007, An
Element of Controversy: The Life of Chlorine in Science, Medicine,
Technology and War, London: British Society for the History of
Science.
Debus, A.G.: 1967, ‘Fire Analysis and the
Elements in the Sixteenth and the Seventeenth Centuries’, Annals of
Science, 23, 127-47.
De Luc, J.A.: 1803, Introduction à
la physique
terrestre par les fluides expansibles; précédée de
deux mémoires sur la
nouvelle théorie chymique, considérée sous
différens points de vue.
Pour servir de suite et de développement aux Researches sur les
modifications de l’atmosphère, 2 vols., Paris: la Veuve
Nyon; Milan: J. Luc Nyon.
Donovan, A. (ed.): 1988, The Chemical
Revolution: Essays in Reinterpretation, Osiris (2nd
Series), 4.
Golinski, J.: 1992, Science as Public
Culture: Chemistry and Enlightenment in Britain, 1760-1820,
Cambridge: Cambridge University Press.
Gough, J.B.: 1988, ‘Lavoisier and the
Fulfillment of the Stahlian Revolution’, Osiris (2nd Series), 4,
15-33.
Guerlac, H.: 1975, Antoine-Laurent
Lavoisier: Chemist and Revolutionary, New York: Charles Scribner’s
Sons (based on Guerlac’s entry on Lavoisier in the Dictionary of
Scientific Biography).
Holmes, F.L.: 1971, ‘Analysis by Fire and
Solvent Extractions: The Metamorphosis of a Tradition’, Isis, 62,
128-48.
Holmes, F.L.: 2000, ‘The ‘Revolution in
Chemistry
and Physics’: Overthrow of a Reigning Paradigm or Competition between
Contemporary Research Programs?’, Isis, 91, 735-53.
Howson, C. (ed.): 1976, Method and
Appraisal in the Physical Sciences, Cambridge: Cambridge University
Press.
Hoyningen-Huene, P.: 2008, ‘Thomas Kuhn and
the Chemical Revolution’, Foundations of Chemistry, 10,
101-15.
Hufbauer, K.: 1982, The Formation of the
German Chemical Community (1720-1795), Berkeley and Los Angeles:
University of California Press.
Kim, M.G.: 2003, Affinity, That Elusive
Dream, Cambridge, MA.: MIT Press.
Kim, M.G.: 2005, ‘Lavoisier, the Father of
Modern Chemistry?’, in: M. Beretta (ed.), Lavoisier in Perspective,
Munich: Deutsches Museum, pp. 167-91.
Kirwan, R.: 1789, An Essay on Phlogiston
and the Constitution of Acids,
new edn., London: J. Johnson (original edition in 1784; French
translation in 1788, with critical notes by Lavoisier et al.; new
English edition in 1789, with an English translation of the French
notes by W. Nicholson, and replies by Kirwan; reprint of the 1789
edition, London: Frank Cass, 1968).
Kitcher, P.: 1993, The Advancement of
Science: Science without Legend, Objectivity without Illusions, New
York and Oxford: Oxford University Press.
Klein, U.: 1994, ‘Origin of the Concept of
Chemical Compound’, Science in Context, 7(2), 163-204.
Klein, U.: 1996, ‘The Chemical Workshop
Tradition and the Experimental Practice: Discontinuities within
Continuities’, Science in Context, 9(3), 251-87.
Klein, U. & Lefèvre, W.: 2007, Materials
in Eighteenth-Century Science, Cambridge, MA.: MIT Press.
Knight, D.M.: 1978, The Transcendental
Part of Chemistry, Folkestone, Kent: Dawson.
Kuhn, T.S.: 1970, The Structure of
Scientific Revolutions, 2nd edn., Chicago: University of Chicago
Press.
Kuhn, T.S.: 1977. ‘Objectivity, Value
Judgment, and Theory Choice’, in: T.S. Kuhn, The Essential Tension:
Selected Studies in Scientific Tradition and Change, Chicago:
University of Chicago Press, pp. 320-39.
Lakatos, I.: 1976, ‘History of Science and Its
Rational Reconstructions’, in: C. Howson (ed.), Method and
Appraisal in the Physical Sciences, Cambridge: Cambridge University
Press, pp. 1-39.
Lavoisier, A.L.: 1789, Traité
élémentaire de chimie, Paris: Cuchet (English
translation by R. Kerr, Elements of Chemistry, 1790; reprint
1965 with an introduction by D. McKie, New York: Dover).
Le Grand, H.E.: 1975, ‘The ‘Conversion’ of
C.-L. Berthollet to Lavoisier’s Chemistry’, Ambix, 22,
58-70.
Lubbock, C.A.: 1933, The Herschel
Chronicle: The Life-story of William Herschel and his Sister Caroline
Herschel, Cambridge: Cambridge University Press.
McEvoy, J.G.: 1988, ‘Continuity and
Discontinuity in the Chemical Revolution’, Osiris (2nd Series),
4, 195-213.
McEvoy, J.G.: 2010, The Historiography of
the Chemical Revolution: Patterns of Interpretation in the History of
Science, London: Pickering & Chatto.
Margolis, H.: 1993, Paradigms &
Barriers: How Habits of Mind Govern Scientific Beliefs, Chicago and
London: The University of Chicago Press.
Mauskopf, S.H.: 1988. ‘Gunpowder and the
Chemical Revolution’, Osiris (2nd Series), 4, 93-118.
Mauskopf, S.H.: 2002, ‘Richard Kirwan’s
Phlogistic Theory: Its Success and Fate’, Ambix, 49,
185-205.
Middleton, W.E.K.: 1965, A History of the
Theories of Rain and Other Forms of Precipitation, London:
Oldbourne.
Morris, R.J.: 1972, ‘Lavoisier and the Caloric
Theory’, British Journal for the History of Science, 6,
1-38.
Multhauf, R.P.: 1966, The Origins of
Chemistry, London: Oldbourne.
Multhauf, R.P.: 1996, ‘Operational Practice
and the Emergence of Modern Chemical Concepts’, Science in Context,
9(3), 241-9.
Musgrave, A.: 1976, ‘Why Did Oxygen Supplant
Phlogiston? Research Programmes in the Chemical Revolution’, in: C.
Howson (ed.), Method and Appraisal in the Physical Sciences,
Cambridge: Cambridge University Press, pp. 181-209.
Nicholson, W.: 1800, ‘Account of the New
Electrical
or Galvanic Apparatus of Sig. Alex. Volta, and Experiments Performed
with the Same’, A Journal of Natural Philosophy, Chemistry and the
Arts, 4, 179-87.
Nordmann, A.: 1986, ‘Comparing Incommensurable
Theories’, Studies in History and Philosophy of Science, 17,
231-46.
Odling, W.: 1871, ‘On the Revived Theory of
Phlogiston’ (Address at the Royal Institution, 28 April 1871), Proceedings
of the Royal Institution of Great Britain, 6, 315-25.
Partington, J.R. & McKie, D.: 1937-39,
‘Historical Studies on the Phlogiston Theory’, Annals of Science,
2, 361-404; 3, 1-58, 337-371; 4, 113-149
(in four parts).
Perrin, C.E.: 1973, ‘Lavoisier’s Table of the
Elements: A Reappraisal’, Ambix, 20, 95-105.
Perrin, C.E.: 1981, ‘The Triumph of the
Antiphlogistians’, in: H. Woolf (ed.), The Analytic Spirit: Essays
in the History of Science in Honor of Henry Guerlac, Ithaca and
London: Cornell University Press, pp. 40-63.
Priestley, J.: 1775-77, Experiments and
Observations on Different Kinds of Air, 2nd edn., 3 vols., London:
J. Johnson.
Priestley, J.: 1790, Experiments and
Observations on Different Kinds of Air, and Other Branches of Natural
Philosophy Connected with the Subject, 3 vols., Birmingham: Thomas
Pearson; London: J. Johnson.
Priestley, J.: [1796] 1969, Considerations
on the Doctrine of Phlogiston, and the Decomposition of Water (and
Two Lectures on Combustion, etc., by John MacLean), New York: Kraus
(original edn., Philadelphia: Thomas Dobson, 1796).
Priestley, J.: 1802, ‘Observations and
Experiments Relating to the Pile of Volta’, (Nicholson’s) Journal
of Chemistry, Natural Philosophy, and the Arts, new series, 1,
198-204.
Priestley, J.: 1803, The Doctrine of
Phlogiston Established and That of the Composition of Water Refuted,
Philadelphia: P. Byrne.
Pyle, A.: 2000, ‘The Rationality of the
Chemical Revolution’, in: R. Nola & H. Sankey (eds.), After
Popper, Kuhn and Feyerabend, Dordrecht: Kluwer, pp. 99-124.
Scheele, C.W.: 1931, The Collected Papers
of Carl Wilhelm Scheele, trans. by L. Dobbin, London: G. Bell &
Sons.
Siegfried, R.: 1964, ‘The Phlogistic
Conjectures of Humphry Davy’, Chymia, 9, 117-24.
Siegfried, R.: 1982, ‘Lavoisier’s Table of
Simple Substances: Its Origin and Interpretation’, Ambix, 29,
29-48.
Siegfried, R.: 1988, ‘The Chemical Revolution
in the History of Chemistry’, Osiris (2nd Series), 4,
34-50.
Siegfried, R.: 1989, ‘Lavoisier and the
Phlogistic Connection’, Ambix, 36, 31-40.
Siegfried, R.: 2002, From Elements to
Atoms: A History of Chemical Composition, Philadelphia: American
Philosophical Society (Transactions of the American Philosophical
Society, vol. 92, no. 4).
Siegfried, R. & Dobbs, B.J.: 1968,
‘Composition: A Neglected Aspect of the Chemical Revolution’, Annals
of Science, 24, 275-93.
Stevenson, W.F.: 1849, The Composition of
Hydrogen, and the Non-Decomposition of Water Incontrovertibly
Established [etc.], 2nd edn., London: James Ridgway.
Sudduth, W.M.: 1978, ‘Eighteenth-Century
Identifications of Electricity with Phlogiston’, Ambix, 25,
131-47.
Thagard, P.: 1990, ‘The Conceptual Structure
of the Chemical Revolution’, Philosophy of Science, 57,
183-209.
Thomson, T.: 1802, A System of Chemistry,
Edinburgh: Bell & Bradfute & E. Balfour.
Wetzels, W.D.: 1990, ‘Johann Wilhelm Ritter:
Romantic Physics in Germany’, in: A. Cunningham & N. Jardine,
(eds.), Romanticism and the Sciences, Cambridge: Cambridge
University Press, pp. 199-212.
Hasok Chang:
Department of History and Philosophy of Science, University of Cambridge, Free School Lane, Cambridge CB2 3RH, United Kingdom; hc372@cam.ac.uk
Copyright © 2010 by HYLE and
Hasok Chang
|


