http://www.hyle.org
Copyright © 2005 by HYLE and Claus Jacob & Adam Walters
HYLE Article |
Risk and Responsibility in Chemical Research: The Case of Agent OrangeClaus Jacob & Adam Walters*
1. IntroductionTwo special issues of Hyle, published in 2001 and 2002, have initiated the debate of ethics within chemical research (see, for example, Davis 2002, Del Re 2001, Kovac 2001, Laszlo 2001, Schummer 2001a/b). Unfortunately, this debate is still in its infancy without the desired impact on research chemists. From a chemist’s perspective, the importance of an ethical discourse might only become apparent when specific examples from everyday chemical research are used to illustrate where and how ethical issues arise that need to be addressed. This paper uses the well-documented historical case of Agent Orange and the related historical twists and turns to discuss ethical questions associated with the synthesis of a novel chemical compound. The next section briefly sets out the
philosophical
background of this study. The third section provides a focused – and
necessarily incomplete– historical overview of Agent Orange, from the
first synthesis of its active ingredients in a research laboratory and
the occurrence of the dioxin contamination to the use of the agent as a
chemical weapon in Vietnam. Sections four to six discuss the questions
of risk and responsibility associated with chemical compounds, from the
synthesis of a hitherto unknown chemical agent to its potential uses
and abuses, using the case of Agent Orange as one major example.[1]
The final section provides a summary and outlook. 2. Risk and responsibilityAs the two special issues of Hyle have illustrated, chemistry provides a fertile ground for different ethical analyses. From a chemist’s perspective, the ethical issues surrounding the synthesis of a genuinely new compound might be the most interesting one. During this process, the chemist develops a method to ‘create’ something intrinsically novel, something that has not existed before. This immediately raises questions related to the risks and responsibilities associated with the compound and its method of synthesis. As far as risks are concerned, we can distinguish between the risk posed by the first chemical synthesis ever and subsequent repetitions (Table 1). The first ever synthesis is a step into the unknown with practical risks for the chemist and workers in the laboratory. History teaches us that such a step can be very dangerous. Generally, it is good chemical practice to perform such an initial synthesis with small quantities and under secure conditions, but the issues related to the remaining risks are rather of a technical than of ethical nature. Similarly, the first batch of the new compound, in small quantities and safely kept, is unlikely to have a major impact on humanity. In contrast, the risks arising from the future availability of a new compound within – and outside – the scientific community as a result of the first ever synthesis are not confined to the research laboratory, and therefore are considerably more difficult to assess and control. These risks associated with the coming into existence of a novel chemical are mainly the result of (a) the future use of larger quantities of the compound, (b) the manufacture process of bulk quantities and (c) the dissemination of the synthetic method in the scientific literature. As we will see in later sections, the issues raised by (a) and (b) are usually dealt with at the level of the chemical industry, not research, and are less important here.[2] In contrast, the risk posed by (c), taken by the research chemist at the point of publishing the synthetic method, is frequently underestimated; both the legal and moral assignment of the resulting responsibilities are vague. This risk is directly relevant to our discussion. Since the ‘creation’ of a new compound is always associated with risks, chemists, in order to continue their research activities, require an ethical model that allows them to assess and ultimately to take such risks. Del Re (2001) has recently addressed issues of risk and responsibility associated with chemical research. As he points out, "in principle, any scientific experiment involves a measure of risk, and therefore of responsibility" – and "responsibility arises from taking the risk" (Del Re 2001). He continues to define the ‘choiceworthiness’ C of an action as the quotient of the expected gain G over the anticipated risk R, with G defined as the product of the desirability D of the positive outcome and its probability, and the risk as the product of the gravity W of the negative outcome and its probability. As we will demonstrate in the case of Agent Orange, this approach encounters some limitations as far as hitherto unknown substances are concerned, especially due to the inability of chemists to estimate the risk associated with chemical impurities. As a consequence, conventional risk assessment might not be enough when dealing with new compounds, and we will use it here in combination with a weak version of the Precautionary Principle. This principle suggests that it is better not to carry out an action that might possibly be very dangerous, as long as counter-evidence is not available. It implies that the synthetic chemist considers a new compound as potentially dangerous and provides initial evidence that it is safe (rather than society having to prove it is unsafe), before the compound or its synthetic procedure are released. Since it transcends conventional principles of risk management and incorporates the notion of uncertainty, the Precautionary Principle is therefore useful in dealing with novel chemical compounds and their impurities. It serves as a constant reminder that, though a risk may not be quantified, there may still be reason to believe that damage may occur. Since it is impossible for chemists to conclusively prove the safety of a new compound in practice, we need to apply a weak version of the Precautionary Principle. For example, an initial estimate of safety and anticipated risk can often be established by looking at chemically similar, already known compounds. Similarly, the presence of risky impurities might be predicted by considering the thermodynamics of a chemical process, such as temperature and pressure control. Nevertheless, the current state of chemistry does not enable chemists to avoid, or fully rationalize the presence of chemical impurities. This scientific weakness is illustrated, for example, by the frequent failure of so-called structure-activity relationships. Although the latter are good initial approximations, small changes in chemical composition or structure, such as optical isomerism, can significantly affect biological activity. As a consequence, no new substance should be considered as harmless unless it has been carefully tested. The combination of risk assessment and a weak version of the Precautionary Principle is able to address two important aspects of chemical research. First, it allows chemists to reject what they might intuitively feel to be ‘immoral’ acts, such as chemical weapons research (high value of W). Second, it allows chemical research, even if associated with risks, to proceed, i.e. it does not rule out all chemical synthesis a priori because of possible risks.
In the following sections, we will use the notion of risk and responsibility to discuss
We will start with a brief historical review
of the
Agent Orange case, with the ethical focus on the issues of risk,
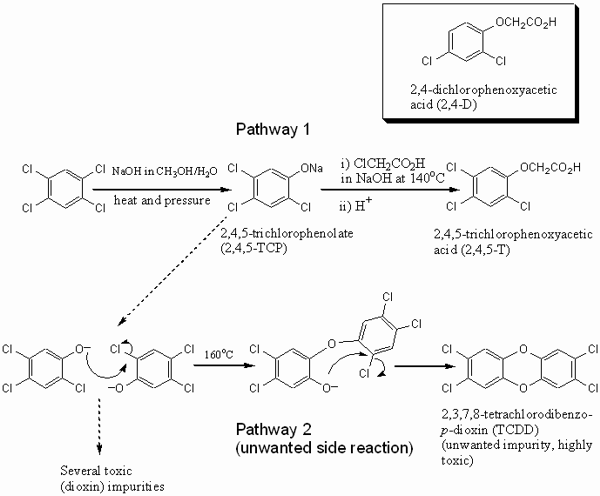
responsibility, and dioxin contamination. 3. A brief history of Agent OrangeThe history of Agent Orange, and its associated dioxin contamination, is important for the discussion of risks and responsibility. It starts with the discovery of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), i.e. the ‘creation’ of the compound in the research laboratory, and then leads to the large scale manufacture of a 2,4,5-T containing herbicide for agricultural use and the manufacture and use of Agent Orange in Vietnam. The first synthesis of 2,4,5-T was published by Robert Pokorny (1941). At the time he was employed at C.B. Dolge Company, and working on pesticides for agricultural use. Four years later, the American Chemical Paint Company patented the use of 2,4,5-T as a weed killer along with a plethora of other forms of halogenated phenoxy monocarboxylic aliphatic acids (including 2,4-dichlorophenoxyacetic acid [2,4-D]) and their esters and salts (US Patent 2390941 from 1945). The Chemical Abstract Service decennial indexes show the subsequent rise of scientific interest in the compound, from 13 related papers between 1937 to 1946 to more than 200 papers between 1947 and 1956. The latter period also sees a rise in applications on a diverse range of plants, and an increase in the number of patents relating to effective delivery techniques. Large scale manufacture of the 2,4,5-T herbicide for agricultural uses at Dow began in 1950 and ceased in 1979, when Dow was the largest company worldwide that manufactured the compound. In the 1950s and 1960s, large-scale manufacture led to the contamination of the herbicide with the poisonous dioxin 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) due to a side reaction (Figure 1) (Heaton 1996). 1,2,4,5-tetrachlorobenzene reacted at high temperatures with hydroxide to form sodium 2,4,5-trichlorophenoxide (2,4,5-TCP, Figure 1, pathway 1). In line with Pokorny’s publication from 1941, this then reacted with chloroethanoic acid at 140°C to obtain 2,4,5-T. Temperature control in both processes was essential, because at 160°C the electron deficient 2,4,5-TCP can undergo a condensation reaction (Figure 1, pathway 2) producing the tetrachloro-substituted dioxin. As it was difficult to obtain uniform temperatures in bulk reaction vessels, 2,4,5-T subsequently contained dioxin contaminants in the order of parts per million (ppm). In subsequent years, this contamination level had to be reduced to below 1 ppm in response to legal requirements. This was achieved by better temperature control or the removal of dioxin from 2,4,5-TCP. At the time Dow stopped the 2,4,5-T manufacture in 1979, the legal upper limit of TCDD contamination was 0.1 ppm in the US and 0.01 ppm in the UK (Hay 1982, p. 9). Health problems associated with 2,4,5-TCP exposure, particularly the development of chloracne, were first noticed during industrial accidents involving the compound, such as at Monsanto (1949), Boehringer in Germany (1952) and at Dow (1964). The link between 2,4,5-TCP, chloracne and an – at the time initially unknown – dioxin impurity in 2,4,5-TCP was established by Karl Schultz in 1957 as a direct response to the Boehringer accident (Kimmig 1957). These developments took place several years before the use of 2,4,5-T in Vietnam. The military use of 2,4,5-T was already considered during World War II and the Korean War, and the British used small amounts during the ‘Malayan emergency’. However, the widespread use of Agent Orange for military purposes began only in 1962, together with five other herbicide mixtures.[3] Under the Defense Production Act of 1950, the US government allowed a number of chemical companies to produce 2,4,5-T for Vietnam, including Dow, Monsanto, Hercules Inc. and Diamond Shamrock.[4] The use of 2,4,5-T in Vietnam had several reasons, most of which were related to the problem of jungle warfare. North Vietnamese soldiers and the Vietcong were adapted to this kind of warfare, using the trees for cover and employing guerrilla tactics. American forces had difficulties to react and their superior firepower rendered immobile in the terrain, so that they sought to remove their enemy’s advantage. Operation Ranch Hand was launched to defoliate the forests and mangroves, and to destroy crops in order to reduce the enemy food supplies. The operation involved at least 19,900 sorties between 1962 and 1971 (Stellman et al. 2003; Hay 1982, p. 147). They employed a range of herbicide mixtures, each known by the color identification band painted on storage barrels. Of these herbicides, Agent Orange was the most widely used blend (45,677,937 liters), consisting of a 50:50 mixture of n-butyl esters of 2,4-D and 2,4,5-T.[5] Agent Orange used in Vietnam was contaminated with varying levels of TCDD, on average around 1.91 ppm or higher (Hay 1982, p. 164; see Figure 1 for chemical structures). The manufacturer’s knowledge about the level of contamination and the toxicity of TCDD at the time of production is hazy. It is complicated by the fact that some herbicide manufacturers purchased already contaminated 2,4,5-TCP. In addition, the determination of dioxins is still complicated and expensive, with only few laboratories able to carry out this kind of trace analysis.
Figure 1: Synthesis of 2,4,5-T. Pathway 1 shows the desired reaction leading to the active ingredient for herbicides. Pathway 2 is a side reaction leading to an unwanted dioxin contamination. In addition to TCDD, a range of other impurities of varying degree of toxicity is formed. Insert upper right: the chemical structure of 2,4-D. As production levels soared to meet military demands, Dow Chemical and other manufacturers attempted to reduce the TCDD levels. Dow also developed methods to measure TCDD levels more accurately. However, dioxin levels in Agent Orange varied dramatically, depending both on manufacturer and production lot. To provide some indication, residues of Agent Orange manufactured at the time were found to contain between 0.05 and 47 ppm dioxin, with considerably higher dioxin concentrations likely in pre-1966 samples, while Dow’s product after the construction of its new plant in 1966 was below 1 ppm (Hay 1982, p. 164). The damaging effects of the TCDD impurity to human health turned out to be considerably more serious than the ones of 2,4-D and 2,4,5,-T, both of which have considerably lower toxicity than TCDD. Although the 2,4,5,-T toxicity is still under debate, the main toxic substance in commercial mixtures of 2,4,5-T based herbicide is clearly not the active ingredient itself, but the dioxin contaminant. For example, neither the West German nor the UK authorities in 1980 were "convinced that 2,4,5-T, if used for the purpose it was intended, presented a health risk" (Hay 1982, p. 177). As a consequence, improved safety of this particular herbicide for commercial use has mostly focused on a reduced dioxin contamination level, and not on 2,4,5-T. This distinguishes 2,4,5-T from many other commercially used chemicals, where the active ingredient itself, and not an impurity, is toxic. In 1969, the history of Agent Orange and dioxins took an unexpected turn. While TCDD was known to cause chloracne since Schultz had published his findings in 1957, a study by the Bionetics Research Laboratories provided evidence that (contaminated) 2,4,5-T was teratogenic in laboratory animals (Courtney et al. 1970). Agent Orange was therefore not only damaging to the Vietnamese ecosystems, but also potentially dangerous to humans. Its use in Vietnam was phased out in 1970/71, and it is now classified as a Class 1 carcinogen.[6] The civil use of 2,4,5-T based herbicides did continue, however, and around 58 tons were still used in the UK in 1980 (Hay 1980, p. 177). On the other hand, the U.S. Environmental Protection Agency (EPA) issued an emergency order to restrict the use of the herbicide for agricultural use in 1979, and launched several attempts in the early 1980s to ban it completely. In 1983, 2,4,5-T based herbicides were withdrawn from the U.S. market and replaced by dicamba and triclopyr.[7] Beginning in the late 1970’s, many American veterans sought compensation for a variety of conditions from chemical companies involved in the Agent Orange manufacture. The responsibility for possible damages caused by the agent was a complex and legally tricky issue. These cases were consolidated into a class action lawsuit that was settled out of court. Since then a limited amount of compensation has been awarded by the government and various lawsuits have been filed against the chemical companies. However, little has been done to provide compensation to Vietnamese people, although there is some evidence suggesting a high rate of birth defects in contaminated areas up to now, which is not surprising because in extreme cases soil samples still exhibit the same concentration of dioxin as Agent Orange (Schecter et al. 2003, Tuan & Phuong 2003). The lack of extensive systematic studies on whether birth defects are indeed elevated in the highly contaminated areas poses a major problem. With the historical development briefly
mapped out,
the next sections will take a closer look at the risk and the
responsibility that emerge at each step of the development of a
compound such as 2,4,5-T, from the first time it is synthesized and the
publication of its synthetic pathway, to its large scale manufacture
and practical application. It should be emphasized from the start that
this analysis aims to stimulate future discussion, and represents just
one possible point of view. 4. Risk and responsibility at the point of inventionAs mentioned before, there is an extensive, and well-documented debate on the legal and moral responsibilities of the manufacturers and users. The initial parts of the history of 2,4,5-T, i.e. its first synthesis, characterization, and publication are often ignored in these discussions. For Example, Hay’s historical review does not mention Pokorny at all. This is unfortunate, since the inventor of a new compound must obviously share some responsibility for the compound, even if the ultimate use is beyond his/her control. This paper therefore focuses on the persons and institutions involved with 2,4,5-T at the beginning of the history of Agent Orange. From a chemist’s perspective, this raises a number of ethical questions that are all too often ignored when just the final use of a compound is considered. The history of Agent Orange is a good example of the chain of events that leads from the discovery to the application of a new chemical. Along the line, there are several actors who might share responsibility for the compounds involved (Table 1). These include (a) Pokorny, the research chemist who discovered 2,4,5-T around 1941, (b) the scientific journal publishing the synthesis of 2,4,5-T in 1941, (c) the company who patented the use of the chemical as herbicide in 1945, (d) the companies and their chemical engineers manufacturing Agent Orange, (e) the American government approving the use of the compound in warfare and (f) the US Air Force/RVN actually using Agent Orange as a tactical chemical weapon. At the point of invention, Pokorny, the synthetic chemist, was the first person involved with 2,4,5-T. It is therefore necessary to ascertain his responsibility for the risks associated with the compound.[8] Schummer has argued that the synthetic chemist developing a new substance will always have some general responsibility for it, as the "first synthesis is the causal step for its existence" (Schummer 2001b). No matter if this is where the chemist’s active relationship with the substance ends, and if the damage is caused by the hands of others, without the initial causal step no damage could have occurred. Although this notion of responsibility does not yet include a moral judgment, it implies the obligation of the chemist to respond to moral questions and accept the standards of a moral discourse. That this may seem bizarre to the average chemist is not surprising. "The fact that the internal norms of [the chemical community] are not in agreement with general moral standards shows that the whole community do not recognize their general moral responsibility and wrongly consider their activity as morally neutral" (Schummer 2001b). Although this is a strong statement, it is indeed curious to realize that research chemists inventing and publishing novel compounds are hardly ever asked by their peers to provide even the most basic information about the potential dangers that might result from these agents. As might therefore be expected, and is normal chemical practice, Robert Pokorny’s paper from 1941 entitled ‘Some chlorophenoxyacetic acids’ considers his new compounds in the light of successful versus unsuccessful synthesis, not under the moral categories of right and wrong. His quarter page report in the ‘New Compounds’ section of the Journal of the American Chemical Society provides the experimental details to successfully make 2,4-D and 2,4,5-T with yields on the 5 g scale and some analytical data. There is neither an introduction to this work, nor a discussion or conclusion. Pokorny does not indicate any future work that could be done with these new compounds, or any potential applications – or safety implications – 2,4,5-T might have. This does not imply that Pokorny himself was not aware of possible applications. His research at the company was clearly directed toward novel herbicides, and it is likely he considered commercial uses, even if he did not explicitly state them in his publication. The act of the first ever synthesis of these compounds, the ‘creation’, is described from a scientific perspective. At the point of invention of 2,4,5-T, the question of responsibility for the ‘creation’ is not raised, and it is doubtful that many chemists in Pokorny’s situation would consider such a synthetic report from an ethical perspective. It should be mentioned, however, that chemists do speculate about the potential benefits of their novel compounds. They do consider possible consequences, and claim responsibility for future applications, be it mostly for the beneficial ones. For the sake of argument, let us therefore assume that Pokorny would have accepted moral responsibility for the risks associated with his compound. This does not mean, of course, that Pokorny would have to accept responsibility for every use of it or its impurities. Nevertheless, he could have performed a risk assessment along the lines suggested by Del Re. Pokorny was obviously aware of the potential agricultural use of 2,4,5-T as a herbicide. Since he could neither have foreseen the TCDD contamination that occurs when his reaction is scaled up, nor the dangers TCDD poses, nor the use of his new compound in Vietnam, his risk assessment in 1941 to determine the choiceworthiness of making 2,4,5-T would therefore have been very favorable, i.e. the desired benefits of 2,4,5-T as a herbicide would have by far outweighed the known risks associated with the compound.[9] For the same reason, the ethical framework used here would not ascribe any major negative moral judgment upon him. As already mentioned, it is curious, however, that in practice a proper risk assessment on new compounds is hardly ever performed by the inventor before the compound is published. In this context, let us look once more at the case of Agent Orange. Any risk assessment made for 2,4,5-T in 1941 would have been severely flawed by the lack of information about the occurrence and implications of the TCDD contamination. In fact, Pokorny does not mention impurities in his publication, and it will be very difficult to find any synthetic chemistry publications that explicitly discuss the content and the nature of impurities in a new compound.[10] This in itself is an interesting aspect of synthetic chemistry, where numerous ‘unidentified’ compounds are regularly ‘created’ and distributed as impurities. As far as the ethical discussion is concerned, the presence of such chemical impurities in compounds has serious implications for the risk assessment of novel chemicals. While chemists might be able to draw some conclusions about their desired new compound based on similarities with known chemicals, they would initially be completely in the dark regarding the properties of undesired, unknown impurities that are beyond their control – and occur in compounds in an uncontrolled, almost random manner. As the TCDD contamination has shown, minute amounts of impurities (in the ppm range) can completely alter the toxicity of a 99.9999% pure substance, as long as the impurities are a few million times more toxic then the pure compound. In a biological context, the risk posed by impurities is even more serious, since many toxins undergo bioaccumulation in organisms, resulting in biomagnification of their concentrations by thousands or even millions in food chains (Southgate & Aylward 2002). While Pokorny might have wanted to consider the toxicity of his sample of 2,4,5-T (which might have contained some TCDD) in a few bioassays to rule out major safety risks in handling the compound, he would have been neither able to anticipate the conditions under which impurities might be formed, nor the exact nature and toxicity of these unknowns. Even worse, the impurities in 2,4,5-T might actually have resulted from using contaminated 2,4,5-TCP, in which case the inventor of that compound, and not Pokorny, might ultimately share responsibility for the TCDD contaminant in 2,4,5-T. Although TCDD and other dioxins were later identified as impurities in Agent Orange, other impurities remain unknown, and some might even contain ‘yet unknown’ compounds, as was indeed initially the case with dioxin in Schultz’s studies. Schummer has recently shown that the increase of knowledge that comes with the synthesis of a new compound is smaller than the non-knowledge generated at the same time (Schummer 1999/2001b). The notion of non-knowledge also applies to such unknown impurities and dramatically complicates risk assessment and the issue of responsibility. For example, how could a chemist like Pokorny be held responsible for the effects of impurities that he was unaware that they even existed, or which were possibly transferred from an impure starting material? In any case, Pokorny’s original publication made 2,4,5-T and 2,4-D available to the chemical community worldwide without a basic understanding if it is safe or toxic, carcinogenic or explosive. Again, Pokorny acted well within accepted current chemical practice, where the inventor of a new compound is not expected to show that the invention is safe. Admittedly, he could have discussed 2,4,5-T in the light of similar compounds known to have herbicidal properties, and provided a basic ‘risk assessment’ based on analogy, but this is difficult and not normally required from chemists at the point of invention. By publishing the synthetic method, however, Pokorny in principle made 2,4,5-T available to everyone.[11] This kind of chemical proliferation by publication is frequently ignored. Since it is, however, much less controlled than the shipment of substances, the main risk associated with the ‘creation’ of a new compound might well reside with the proliferation of the associated chemical knowledge, and not with the few grams of new compound actually made and safely stored in a secure laboratory. Although this might sound bizarre, Pokorny therefore shares direct responsibility for the worldwide, indirect proliferation of an untested compound. For the inventor, this responsibility might outweigh any responsibility for the subsequent manufacture or use of the agent as discussed above. The inventor of a new compound is in a moral dilemma caused by potentially conflicting values. On the one hand, the scientific codex requires the publication of results, so that other scientists can repeat, test, and benefit from the new compound. On the other hand, the compound might well be unsafe and cause significant damage – possibly even to the colleagues trying to repeat the synthesis. While here is not the place to discuss this dilemma in detail, chemists should become aware of this issue, and try to address it in the future.[12] The question of uncontrolled proliferation of
chemicals through publication in scientific journals brings us to
another, rather unsuspected carrier of responsibility for a new
chemical compound – the scientific journals that disseminate synthetic
knowledge. 5. Publishing as proliferationThe inventor of a new compound shares responsibility for the dissemination of the synthetic procedure, including the almost uncontrolled indirect proliferation via online journals, Internet postings, and free online services such as Medline. It is obvious that without the dissemination of Pokorny’s synthetic protocol in the Journal of the American Chemical Society, 2,4,5-T would not have become ‘freely available’ to chemists outside Pokorny’s own organization at that particular time. This does not rule out, of course, that someone else might have invented the compound soon thereafter, but this is beside the point. As a consequence, a journal such as the Journal of the American Chemical Society might share moral responsibility for the potentially thousands of kilograms of novel compounds being manufactured on a large scale by whomever is willing to do so. This ethical consideration is in agreement with the fact that journals frequently request the copyrights on manuscripts containing synthetic protocols before publication. There is, however, little in the Journal of the American Chemical Society’s current ‘Ethical Guidelines to Publication of Chemical Research’ that would address the ethical issues raised here. The latter deal with good scientific practice and how to provide sound and reliable data, a rather different area of ethics and chemistry. As for safety issues, the synthetic procedure and analytical data of novel compounds can be published without further warning. In contrast, some journals have recently begun to ask authors to explicitly address such ‘safety issues’ as part of their publications. For instance, the Journal of Agricultural and Food Chemistry’s ‘Scope, Policy and Instructions for Authors Guidelines’ says: Safety: Authors are required to call special attention, in both their manuscripts and their covering letter, to safety considerations such as explosive tendencies, special precautionary handling procedures, and toxicity. Such requests might well stimulate a basic discussion of the risks associated with a novel compound. Nevertheless, neither the authors of the manuscript, i.e. the inventor(s) of the compound, nor the publisher take responsibility for the risks associated with the new agent. From a chemist’s perspective, this might seem normal, and is in line with Schummer’s observation that chemists do not recognize the wider responsibility for their discoveries. Let us therefore briefly consider how chemically good practice might in the future be aligned with ethically good practice. As the discussion of chemical impurities has shown, a reliable risk assessment for a novel chemical compound is virtually impossible, but some suggestions based on similar chemicals might be possible. As a consequence, a weak version of the Precautionary Principle should be applied when dealing with new compounds – and associated unidentified impurities. The anticipated risk can, in certain cases, be described by looking at chemically similar, already known compounds. In other cases, especially when impurities are present and conclusions by analogy are impossible (e.g. compare 2,4-D and 2,4,5-T), a basic toxicological screen might be required. The latter would also detect the toxicity caused by unknown impurities. Although it seems to be the most suitable
approach
toward dealing with the risks associated with novel compounds, the
Precautionary Principle is hardly applied in everyday chemical
research. Of course, most synthetic chemists are unable to carry out
full toxicological evaluations of each of their new compounds, and to
demand such studies would be detrimental to chemical research.
Nevertheless, publishers, as a matter of precaution, should follow the
lead taken by the Journal of Agricultural and Food Chemistry
and others and demand at least a brief discussion of the
potential risks associated with a new compound, such as toxicity or
potential environmental impact.[13] 6. Manufacturers and usersResponsibility of the manufacturer is a complicated mix of regulatory, legal, and moral issues that cannot be fully discussed here. As in the case of Agent Orange, the manufacturer of a chemical shares responsibility for the resulting product, but no longer for the ‘creation’ or dissemination of the synthetic protocol. This is a rather important distinction frequently missed in ethical reflections on chemistry and the chemical industry. Since we are more concerned here with responsibility as part of invention, and since there is ample literature on the legal and ethical issues related to the manufacturers and users of Agent Orange, this section will briefly focus on the issue of unknown risks, and ask if it possible to deflect the responsibility for impurities. On its website, Dow Chemical denies responsibility for the damages caused by Agent Orange and assigns this responsibility to the users of Agent Orange, i.e. the U.S. and Vietnamese governments. As a nation at war, the U.S. government compelled a number of companies to produce Agent Orange under the Defense Production Act. Companies supplying Agent Orange to the government included The Dow Chemical Company, Monsanto Company, Hercules Inc., Diamond Shamrock Chemicals Company, Uniroyal Inc., Thompson Chemical and T-H Agriculture and Nutrition Company. […] The U.S. military had sole control and responsibility for the transportation of Agent Orange to Vietnam, and for its storage once the defoliant reached Vietnam. The U.S. military controlled how, where, and when Agent Orange would be used. […] War damages people, lives, and the environment. Nations, and the militaries of nations, are responsible for war. The U.S. government and the Vietnamese government are responsible for military acts in Vietnam and the use of Agent Orange as a defoliant. The manufacturers feel that in 1984 they took part in a good-faith settlement aimed at healing and bringing closure to this issue. Any future issues involving Agent Orange should be the responsibility of the respective governments as a matter of political and social policy.[14] This line of arguments shows that the manufacturer denies responsibility for the use of the chemical in Vietnam, referring to the government’s actions. It also uses the controversial idea that the manufacturer is not required to prove the product’s safety, but that the injured party must be able to ‘prove causation’. Our discussion on who shares responsibility for the impact of a new chemical such as 2,4,5-T on society has now reached the point where neither the inventor, nor the publisher, nor the manufacturer seems required to accept responsibility. In the case of Agent Orange, this debate is complicated by the fact that the damage was caused by a chemical impurity. Since no one involved with Agent Orange intended the harm caused by TCDD, or initially knew of the dangers caused by the impurity, this raises a set of very different ethical issues. For example: While the U.S. and Vietnamese governments might be responsible for using Agent Orange as an herbicide, can the manufacturers also hold them responsible for the effects of the TCDD impurities in the herbicide? Even if Dow was not fully aware of the contamination and its associated risks, would it not have been the manufacturer’s responsibility to check the quality, i.e. chemical composition, of their product? Dow’s comment on this issue is rather instructive. Much of the source of the resulting public controversy over Agent Orange was an unwanted trace impurity that was present in one of the product’s ingredients. The unwanted contaminant was the dioxin compound 2,3,7,8-tetrachlorodibenzo-para-dioxin, commonly known as 2,3,7,8 or dioxin. It should be noted that dioxin was not a commercial product, but rather was an unavoidable manufacturing process contaminant in the 2,4,5-T process. [Ibid.] The description of dioxin in Agent Orange as "unwanted contaminant" that was "unavoidable" as part of the manufacturing process seems to deflect responsibility and moral judgment from the manufacturer. First, the company did not ‘want’ the presence of dioxins, and thus acted without bad intentions. Second, the dioxin contamination could not be avoided because of the chemistry of the manufacturing process. From an ethical perspective, Dow’s arguments are hardly convincing. The lack of intention to cause bad consequences and the lack of knowledge about the risks do not rule out responsibility. In line with the general concept of responsibility (Schummer 2001b), we therefore propose that the manufacturer of a chemical compound must accept a shared responsibility for both, the alleged (pure) compound and the impurities usually associated with the compound. In practice, this would require the manufacturer to estimate the nature, amount, and impact of impurities in a number of samples. While this could still not rule out a significant fluctuation in impurities between samples, it would provide at least some probability for a reliable safety and risk assessment. Furthermore, the manufacturer is able to design a suitable manufacturing process for a chemical. In the context of Agent Orange, alternative synthetic routes for 2,4,5-T, avoiding the presence of 2,4,5-TCP, exist and could have been explored by the chemical industry to avoid the presence of TCDD. Responsibility for the decision to produce Agent Orange along the hazardous, yet convenient 2,4,5-TCP route, therefore rests fully with the manufacturers and their chemical engineers. Within this context, the matter of impurities is complicated by the different quality standards industrial chemicals have to comply with. Pharmaceutical products, such as drugs, for example, have to comply with considerably higher standards of purity than agricultural products, such as herbicides. As a consequence, there are both ethical and regulatory questions to be addressed. In any case, the manufacturer clearly shares some responsibility, and there are now stringent rules that govern the commercialization of substances, such as the Toxic Substances Control Act in the U.S.[15] In many cases, however, the burden of responsibility for proving that a substance is toxic still lies with society (consider the roles of the Environmental Protection Agency and the British Environment Agency). Overall, the case of Agent Orange points
toward shared responsibility, with the inventor of 2,4,5-T (i.e.
Pokorny), his employers, the publishers of his 1941 paper, and the
manufacturers of the (contaminated) herbicide sharing different degrees
of responsibility for the chemical compound and its dissemination.
Though ethical responsibility for compounds may be traced back to
creators and manufacturers, this responsibility in no way detracts from
that of the user. The responsibility of the U.S. government is
therefore as apparent as the responsibility of the US Air Force/RVN for
using the product. There is also a long philosophical dialogue about
ethics and war. However, this is not the place to discuss these issues
in detail.[16] 7. ConclusionThe issues surrounding Agent Orange, such as the risk associated with the generation of non-knowledge, e.g. caused by impurities, and the indirect proliferation of chemical compounds by publication, have raised important, pressing questions that chemists need to address. In the short term, it is unrealistic to expect synthetic chemists to perform in depth risk assessments for all of their new compounds. Nevertheless, the assignment of responsibility for the dissemination of untested substances might make chemists more aware of the implications of their work, and make them more cautious when dealing with new substances and the knowledge and non-knowledge that come with them. The attempts of Dow Chemical Company to shift responsibility for its contaminated herbicide first to the user and then to the chemical reaction itself show how controversial this issue is in practice. Traditional concepts of risk assessment might not easily be applied and the question of how a chemist can be held responsible for the action of impurities he/she is not aware of clearly requires further investigation. From the outset, this case study has
primarily
aimed at identifying ethical issues in a real life chemistry context,
and to stimulate further debate. It is hoped that an ensuing discussion
of this case study will make chemists more aware of their
responsibility for new chemicals. Only then will it be possible for
chemists to follow philosophers and ‘recognize their general moral
responsibility’ and ‘align their internal norms with general moral
standards’. Notes[1] This discussion focuses on risks associated with chemicals. It should be noted that many chemicals have thoroughly positive effects on human health and the environment. Some of the considerations, such as the proliferation of chemical knowledge, apply to both. [2] They might, for example, include the use of a chemical as a drug or air pollution as the result of the manufacturing process. [3] See Butler 2003a/b; for excellent historical reviews of this and related topics, see also Hay 1982 and Gough 1987. [4] Although the following discussion mainly focuses on Dow, companies like Monsanto and Diamond Shamrock share a similar, if not greater, responsibility for the manufacture of Agent Orange. [5] Both 2,4,5-T and 2,4-D were generally applied as either esters or salts of the acids. Long chain (hence low volatility) esters were applied as an oil emulsion and were more toxic to plants than the water-soluble salts. The ester’s toxicity is due to the lipophilic compound being readily taken up by cuticular lipids. Surfactants were sometimes added to mixtures to increase effectiveness by reducing run-off and by softening lipids (Hassall 1982). In plants esters are hydrolysed back to the biologically active phenoxy acetic acids by carboxylesterase enzymes. The exact modes of action vary, but both herbicides cause lethal, uncontrollable, and grossly distorted plant growth when applied in the correct dosage. [6] TCDD causes, among others, soft-tissue sarcoma, non-Hodgkin lymphoma, Hodgkin disease, chronic lymphocytic leukemia, diabetes, chloracne, birth defects, fetal death, reduced fertility (in both sexes), modulation of hormone levels, and potentially a wide range of cancers (USEPA 1994, Institute of Medicine 2000, Tuan & Phuong 2003). TCDD is categorized as carcinogenic to humans by the International Agency for Research on Cancer (IARC) and has recently been subject to various legislative controls (Stringer & Johnston 2001). Apart from Agent Orange, dioxins have also played a role in several major accidents, such as in Coalite in the UK (1968) and in Seveso in Italy (1976). Only 39 g of TCDD escaped in Coalite, resulting in a soil concentration of 400 ppb. 1.3 kg TCDD was released in Seveso, leading to soil concentration up to 235 m g dioxin per square meter, and the death of thousands of animals (Stringer & Johnston 2001, pp. 305-34). [7] See http://www.sciencedaily.com/encyclopedia/herbicide. [8] Importantly, this is not to pass a moral judgment on the inventor. As we will see later on, taking responsibility for a new compound does not mean taking the blame for all future uses of it. [9] Pokorny’s samples of 2,4,5-T might well have contained dioxin impurities. The presence and effects of such dioxins, if present, would have gone largely unnoticed. Laboratory chemicals are, however, frequently of higher purity than chemicals produced on an industrial scale, due to better reaction control and purification methods. [10] Some chemists do provide percentage purities of new compounds in their publications, but this is not always the case. In addition, impurities frequently vary from synthesis to synthesis, and might consist of yet unknown substances. [11] Any skilled chemist reading Pokorny’s paper would be able to manufacture 2,4,5-T. [12] Related issues, such as the proliferation of chemicals and commercial interests have recently been discussed by Laszlo (2001) and Kovac (2001). [13] The other American Chemical Society (ACS) journal mentioned here, J. Am. Chem. Soc., does not demand this kind of safety information yet. [14] http://www.dow.com/commitments/debates/agentorange/background.htm, last visited 10 Oct. 2005. [15] As mentioned earlier, the dioxin contamination resulted as part of the 2,4,5-TCP synthesis. In many cases, the latter was purchased, not manufactured, by the herbicide company. The responsibility for ensuring the safety of 2,4,5-T might therefore rest with both, the 2,4,5-T and the 2,4,5-TCP manufacturers. [16] Some of the
ethical issues
related to the use of Agent Orange in Vietnam are raised by Hay (1982).
Issues of ethics and war are addressed by Coates (1997). References Butler, D.: 2003a, ‘Flight records reveal full extent of Agent Orange contamination’, Nature, 422, 649. Butler, D.: 2003b, ‘Agent Orange health investigation stuck at square one’, Nature, 422, 793. Coates, A.J.: 1997, The Ethics of War, Manchester UP, Manchester. Courtney, K.D.; Gaylor, D.W.; Hogan, M.D.; Falk, H.L.; Bates, R.R. & Mitchell, I.: 1970, ‘Teratogenic evaluation of 2,4,5-T’, Science, 168, 864-6. Davis, M.: 2001, ‘Do the Professional Ethics of Chemists and Engineers Differ?’, Hyle – International Journal for Philosophy of Chemistry, 8, 21-34. Del Re, G.: 2001, ‘Ethics and Science’, Hyle – International Journal for Philosophy of Chemistry, 7, 85-102. Gough, M.: 1987, Dioxin, Agent Orange, Plenum Press, New York. Hassall, K.A.: 1982, The chemistry of pesticides their metabolism, mode of action and uses in crop protection, Macmillan, London. Hay, A.: 1980, ‘Red faces (and hot tempers) on 2,4,5-T’, Nature, 286, 97. Hay, A.: 1982, The Chemical Scythe, Plenum Press, New York and London. Heaton, A.: 1996, ‘Pesticides’, in: Heaton, A. (ed.), The Chemical Industry, Blackie Academic & Professional, London, pp. 238-43. Institute of Medicine: 2000, Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides, Washington, DC [www.iom.edu] Kimmig, J. & Schultz, K.H.: 1957, ‘Chlorierte aromatische zyklische Aether als Ursache der sogenannten Chlorakne’, Naturwissenschaften, 44, 337-8. Kovac, J.: 2001, ‘Gifts and Commodities in Chemistry’, Hyle – International Journal for Philosophy of Chemistry, 7, 141-53. Laszlo, P.: 2001, ‘Handling Proliferation’, Hyle – International Journal for Philosophy of Chemistry, 7, 125-40. Schecter, A.; Quynh, H.T.; Pavuk, M.; Papke, O.; Malisch, R. & Constable, J.D.: 2003, ‘Food as a source of dioxin exposure in the residents of Bien Hoa City, Vietnam’, Journal of Occupational and Environmental Medicine, 45, 781-8. Pokorny, R.: 1941, ‘Some Chlorophenoxyacetic Acids’, Journal of the American Chemical Society, 63, 1768. Schummer, J.: 1999, ‘Coping with the Growth of Chemical Knowledge. Challenges for Chemistry Documentation, Education, and Working Chemists’, Educación Química, 10, 92-101. Schummer, J.: 2001a, ‘Editorial’, Hyle – International Journal for Philosophy of Chemistry, 7, 83-4. Schummer, J.: 2001b, ‘Ethics of Chemical Synthesis’, Hyle – International Journal for Philosophy of Chemistry, 7, 103-24. Southgate, C. & Aylward, A.: 2002, ‘Environmental Ethics: Further Case-Studies’, in: Bryant, J.; La Velle, L.B. & Searle, J. (eds.) Bioethics for Scientists, Wiley, Chichester, pp. 73-83. Stellman, J.M.; Stellman, S.D.; Christian, R.; Weber, T. & Tomasallo, C.: 2003, ‘The extent and patterns of usage of Agent Orange and other herbicides in Vietnam’, Nature, 422, 681-7. Stringer, R. & Johnston, P.: 2001, Chlorine and the Environment: An Overview of the Chlorine Industry, Kluwer, Dordrecht. Tuan, V.M. & Phuong, N.T.N.: 2003, ‘The effects of Dioxin Exposure on the Occurrence of Birth Defects: A Case Control Study at Tudu Hospital, Organohalogen Compounds, 60, 1-3. U.S. Environmental Protection Agency: 1994, Health Assessment Document for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds III of III, EPA/600/BP, Washington, DC [www.epa.gov]. Claus Jacob (correspondence author): |
|||||||||||||||||||||||||||